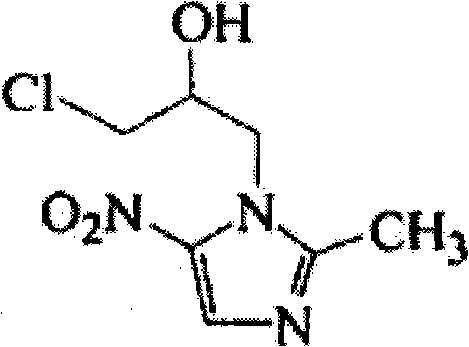
Ornidazole sustained-release dropping pill and preparation method thereof
A technology for sustained-release dropping pills and ornidazole, which is applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. Problems such as allergic reactions or adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] First group:
[0027] Based on the total weight of 100g, weigh the matrix PEG1500 20%, PEG6000 10%, PEG4000 40%, stearic acid 10%, glyceryl monostearate 10%, raw material ornidazole 10%; place the matrix in a heating container Heat and stir to make it melt, add the corresponding proportion of ornidazole, stir fully, and under the condition of heat preservation, drop the molten or mixed liquid medicine into the condensation column filled with simethicone oil, wherein, the The temperature is 55°C, the temperature of the upper part of the condensate is 30°C, and the temperature of the bottom is -4°C; take it out after forming.
[0028] The obtained product has a 2-hour cumulative release percentage of 35-55%, a 6-hour cumulative release percentage of 65-83%, a 10-hour cumulative release percentage of 73-96%, good hardness and roundness.
[0029] Second Group:
[0030] Based on the total weight of 100g, weigh 30% of the matrix PEG1500, 30% of PEG4000, 10% of polyvinylpyrr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com