Load type stephanoporate metal organic compound hydrogen storing material
A technology of organic compounds and porous metals, applied in hydrogen separation, other chemical processes, chemical instruments and methods, etc., can solve problems such as unsatisfactory and complicated preparation methods, and achieve good hydrogen absorption and desorption, simple preparation process, and simple synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
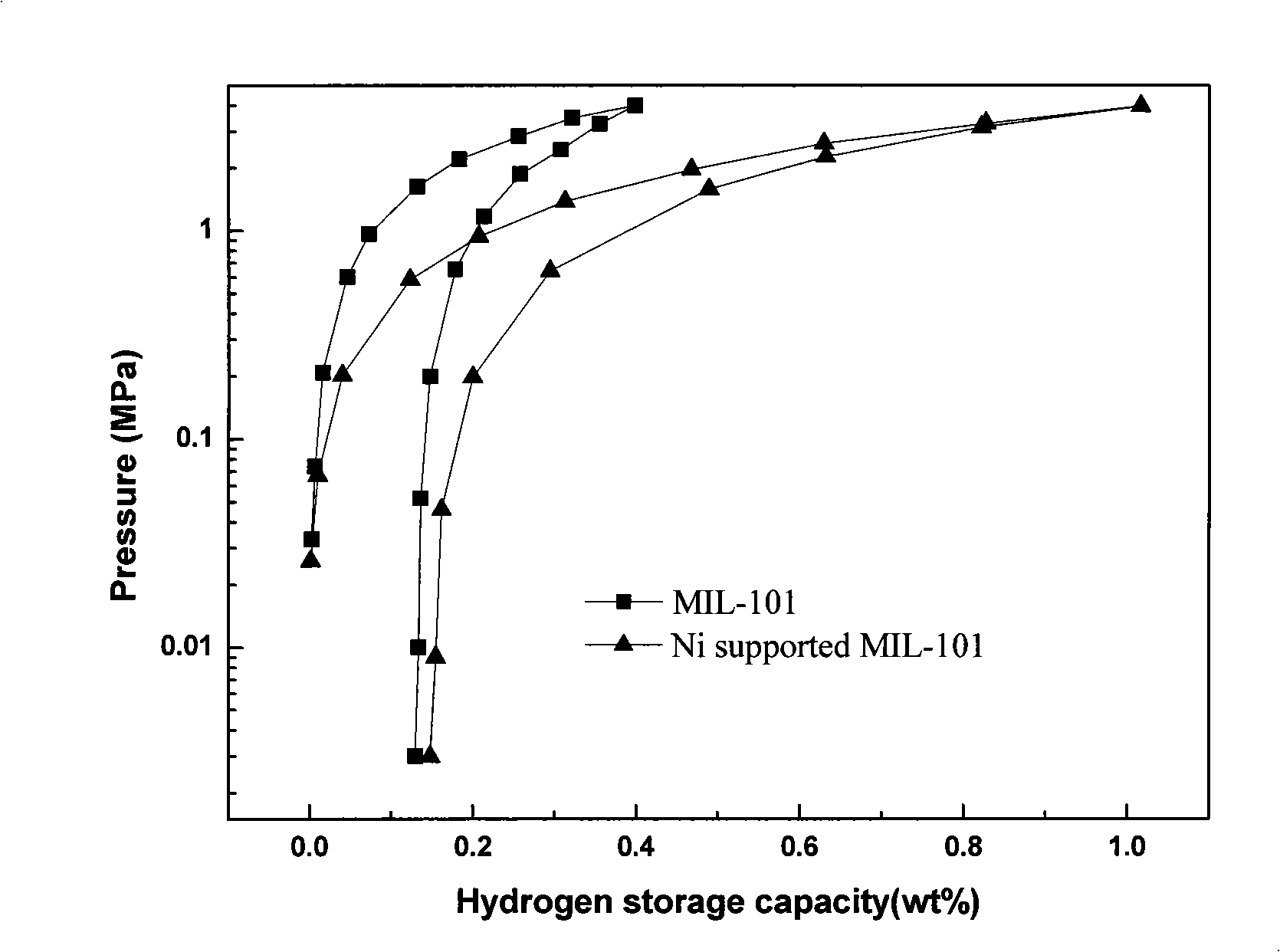
[0027] 1) References (G.Ferey, C.Mellot-Draznieks, C.Serre, F.Millange, J.Dutour, S.Surble and I.Margiolaki, Science, 2005, 309, 2040.) Synthesis of porous metal organic compound MIL -101 (Cr 3 F(H 2 O) 2 O[(O 2 C)-C 6 h 4 -(CO 2 )] 3 ·nH 2 O, n~25): Weigh 4.0g Cr(NO 3 ) 2 Dissolve in 50ml deionized water, after fully dissolving, weigh 1.6613g terephthalic acid and mix it with the solution, then add 10 drops of HF solution dropwise to the mixed solution, then move the mixed solution into a 100ml stuffy tank type stainless steel autoclave (poly Tetrafluoroethylene lining), crystallized in a synthetic oven at 220°C for 10h, cooled to room temperature, filtered and washed the product, dried at 100°C, and dissolved the dried product in 50ml N,N-dimethylformamide , filtered and washed, and dried overnight at 100°C to obtain the target product MIL-101.
[0028] 2) Modified MIL-101 by equal volume impregnation method, weighed 0.1g NiCl 2 , dissolved in 1.2ml deionized wat...
Embodiment 2
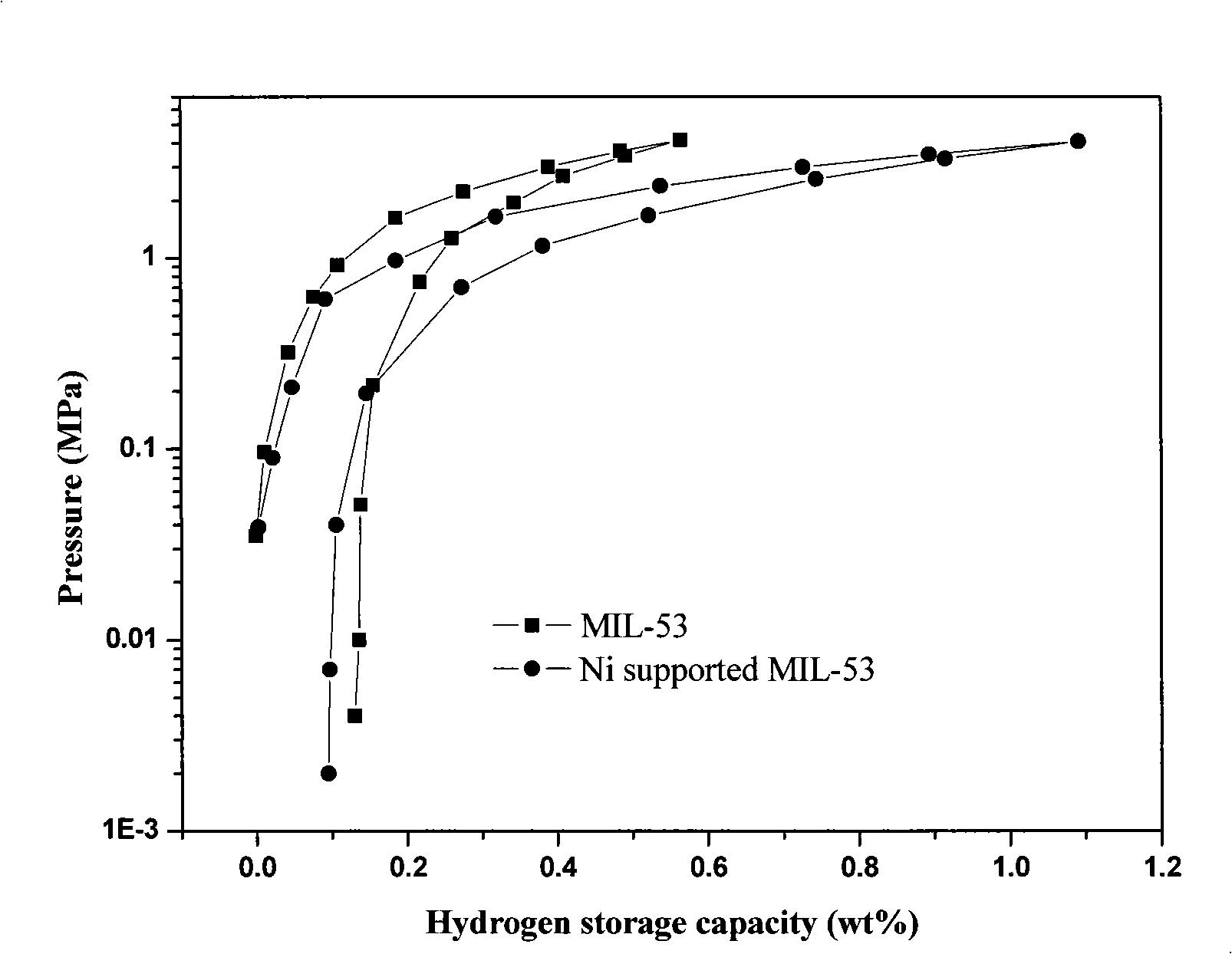
[0035]1) References (T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Ferey, Chem-eur J, 2004, 10, 1373-1382. ) to synthesize porous metal-organic compound MIL-53(Al(OH)[O 2 C-C 6 h 4 -CO 2 ][HO 2 C-C 6 h 4 -CO 2 ] 0.70 ): Weigh 15.6g Al(NO 3 ) 3 Dissolve in 60ml of deionized water, after fully dissolving, weigh 3.456g of terephthalic acid and mix it with the solution, then transfer the mixed solution into a 100ml stuffy stainless steel autoclave (lined with polytetrafluoroethylene), and put it in a synthetic oven at 220 Crystallize at ℃ for 3 days, after cooling to room temperature, filter and wash the product, and dry at 100°C, dissolve the dried product in 50ml of N,N-dimethylformamide, filter and wash, and dry at 100°C overnight. Obtain target product MIL-53.
[0036] 2) Modified MIL-53 by excessive impregnation method, weighed 0.2g NiCl 2 ·6H 2 O, dissolved in 10ml deionized water, weighed 1.0g MIL-53, soaked in the aqueo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com