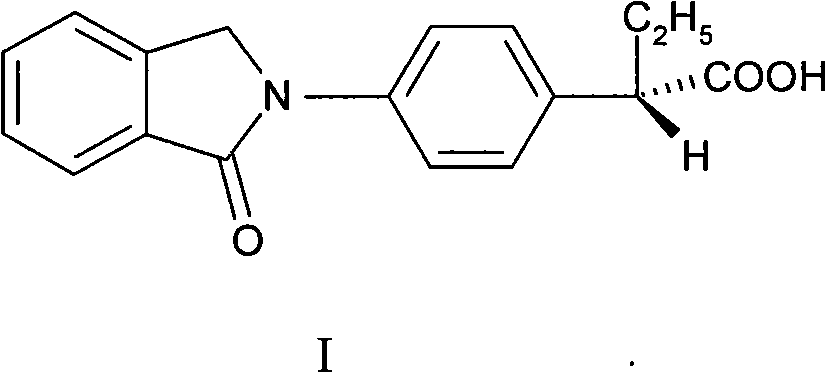
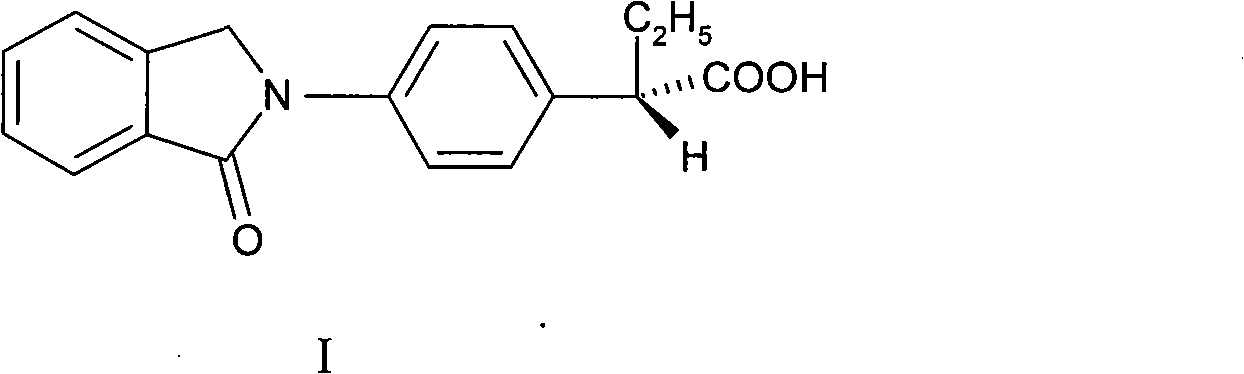
Right-handed indobufen and use for preparing medicament
A technology for dextroindobufen and antiplatelet drugs, which is applied in the field of dextroindobufen and its preparation, and can solve the problems of preparation of a single dextrorotatory optical isomer and the lack of attention to its medicinal value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] The preparation of embodiment 1 dextroindobufen
[0013] Add 50 grams of indobufen, 21.5 grams of (S)-(-)-α-phenethylamine, and 500 ml of ethanol into a 1L reaction bottle, heat to dissolve completely, leave at room temperature overnight for crystallization, filter, wash with ethanol, The resulting solid was recrystallized twice from ethanol, then treated with 2N hydrochloric acid, filtered and washed with water to obtain 11.8 g of dextroindobufen. [α] D =+82.3° (C=1.0, DMF), mp 196-198°C.
Embodiment 2
[0014] The preparation of embodiment 2 dextroindobufen
[0015] Add 50 grams of indobufen, 30.4 grams of (S)-(-)-α-naphthylethylamine, and 500 ml of ethanol into a 1L reaction bottle, heat to dissolve completely, leave at room temperature overnight for crystallization, filter, wash with ethanol, The resulting solid was recrystallized twice from ethanol, then treated with 2N hydrochloric acid, filtered and washed with water to obtain 11.4 g of dexindobufen. [α] D =+81.9° (C=1.0, DMF), mp 196-198°C.
Embodiment 3
[0016] The preparation of embodiment 3 dextroindobufen tablet
[0017] Take 200g of dextroindobufen, add 30g of starch and 15g of lactose, mix well, use 75% ethanol solution to make soft material, granulate with 20 mesh sieve, dry at 60°C, granulate with 20 mesh sieve after drying, add After 3.5 g of sodium carboxymethyl starch and 1.5 g of magnesium stearate are mixed evenly, a No. 9 punch die is used to adjust the tablet weight to 0.25 g / tablet, and the tablets are compressed to obtain final product. The assay result content is 100.2%, and after 6 months of long-term sample retention, it still remains stable. There was no significant change in the detection indicators from the 0 day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com