Bis(8-quinoline diazo amido)-biphenyl, preparation method and application thereof
A technology of nitrogen amino and quinoline, which is applied in the field of derivatives containing bistriazene structure, can solve the problems of triazene reagent interference, etc., and achieve the effects of low cost, enlarged conjugated system, and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1), the diazotization of 8-aminoquinoline
[0023] Add 2mL of concentrated hydrochloric acid and 0.288g (0.002mol) of 8-aminoquinoline into the flask, slowly add 2mL of 0.069g / mL sodium nitrite aqueous solution under stirring at 2°C, and accelerate the stirring reaction for 2h to complete the diazotization.
[0024] 2), the preparation of two (8-quinoline diazoamino)-biphenyl
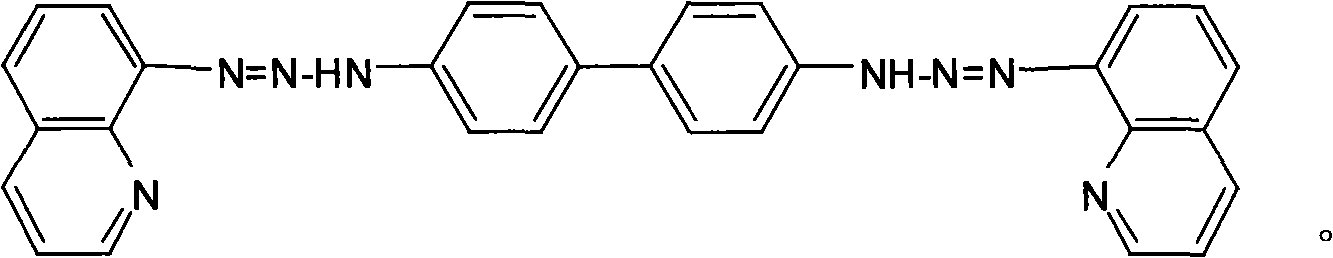
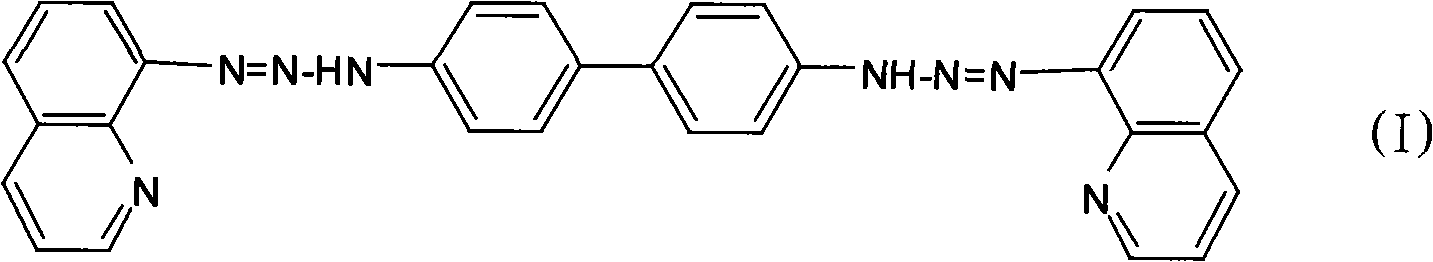
[0025] Dissolve 0.184g (0.001mol) of benzidine in 10mL of ethanol, slowly add the above diazonium salt solution under stirring at 0°C, and adjust the pH of the mixture to 7 with saturated sodium carbonate solution, and react for 2h. Let stand overnight, filter with suction, and wash with water and ethanol solution successively. After drying, recrystallize twice with 95% ethanol to obtain 0.346 g of pure bis(8-quinolinediazoamino)-biphenyl (BQDADB) with a yield of 70%.
[0026] The product was tested by elemental analysis, infrared spectrum and nuclear magnetic resonance spectrum, and it was con...
Embodiment 2
[0028] 1), the diazotization of 8-aminoquinoline
[0029] Add 5mL of a mixed solution of concentrated hydrochloric acid and phosphoric acid, 5mL of water, 0.288g (0.002mol) of 8-aminoquinoline into the flask, slowly add 4mL of 0.069g / mL sodium nitrite aqueous solution under stirring at 0°C, and accelerate the stirring reaction for 2.5h , so that the diazotization is complete.
[0030] 2), the preparation of two (8-quinoline diazoamino)-biphenyl
[0031] Dissolve 0.184g (0.001mol) of benzidine in 10mL of ethanol, slowly add the above diazonium salt solution under stirring at 2°C, adjust the pH of the mixture to 6 with saturated sodium carbonate solution, and react for 2.5h. Let stand overnight, filter with suction, and wash with water and ethanol solution successively. After drying, recrystallize twice with 95% ethanol to obtain 0.386 g of pure bis(8-quinolinediazoamino)-biphenyl (BQDADB) with a yield of 78%.
[0032] The product was tested by elemental analysis, infrared sp...
Embodiment 3
[0034] 1), the diazotization of 8-aminoquinoline
[0035] Add 4mL of concentrated sulfuric acid, 4mL of water, 0.288g (0.002mol) of 8-aminoquinoline into the flask, slowly add 2.5mL of 0.069g / mL sodium nitrite aqueous solution under stirring at 3°C, accelerate the stirring reaction for 1.5h, and make the diazo complete.
[0036] 2), the preparation of two (8-quinoline diazoamino)-biphenyl
[0037] Dissolve 0.184g (0.001mol) of benzidine in 20mL of ethanol, slowly add the above diazonium salt solution under stirring at 0°C, and adjust the pH of the mixture to 6 with saturated sodium carbonate solution, and react for 1.5h. Let stand overnight, filter with suction, and wash with water and ethanol solution successively. After drying, recrystallize twice with 95% ethanol to obtain 0.3 g of pure bis(8-quinolinediazoamino)-biphenyl (BQDADB) with a yield of 60%.
[0038] The product was tested by elemental analysis, infrared spectrum and nuclear magnetic resonance spectrum, and it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com