Method for preparing recombined human amyloid A beta 42 and application thereof
An amyloid and protein technology, applied in the field of fusion proteins, can solve the problems of difficulty in synthesis, increase in cost, application limitations, etc., and achieve the effects of low cost and high protein yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
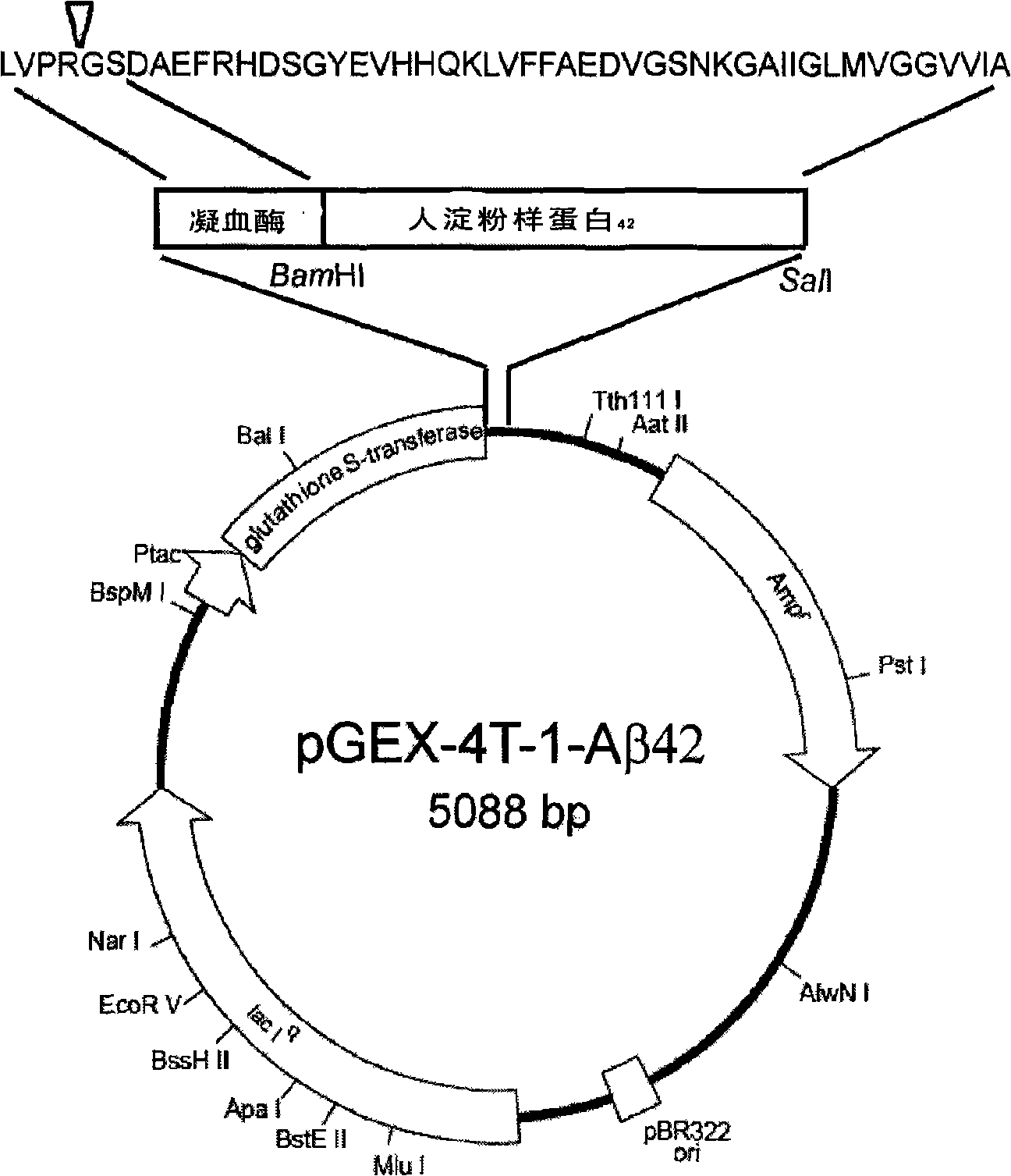
[0029] Example 1: pGEX-4T-1 / Aβ 42 Plasmid construction
[0030] (1) Obtaining cDNA: The total RNA of SH-SY5Y cells from human neuroma blasts was extracted, and the cDNA of SH-SY5Y cells was obtained by reverse synthesis using oligo(dT) as a primer.
[0031] (2) Primer design and PCR: search genbank, based on human Aβ 42 The sequence and the multiple cloning site primers of the vector pGEX-4T-1 were designed.
[0032] upstream primer P 1 : 5'-CG GGATCC GATGCAGAATTCCGACATGACTCAG-3', introduce BamHI restriction site,
[0033] downstream primer P 2 : 5'ACGC GTC GAC CTACGCTATGACAACACCGCCCA-3', introducing a SalI restriction site and a stop codon TAG.
[0034] Using human SH-SY5Y cell cDNA as template for PCR amplification, the product fragment length is 147bp. Amplification conditions: Pfu DNA polymerase, 30 cycles of amplification at 94°C for 30s, 60°C for 30s, and 72°C for 1min.
[0035] (3) Double enzyme digestion and ligation of the PCR product and the vector: BamHI a...
Embodiment 2
[0043] Example 2: GST-Aβ 42 Prokaryotic expression and purification of fusion proteins
[0044] (5) Pre-expression. Transform the expression strain Escherichia coli BL21 with the recombinant plasmid with the identified sequence and correct insertion direction. The molecular weight of the GST protein expressed by the empty vector pGEX-4T-1 is 27KD, GST-Aβ 42 The molecular weight of the fusion protein is about 32KD. Induce with 1mM IPTG at 37°C for 0, 1, 3, 5 hours, thaw the centrifuged cells with 1×SDS lysate, boil at 95°C for 5min, analyze by SDS-PAGE electrophoresis, and make corresponding antibodies (GST and Aβ 42 ) western blot analysis. Both SDS-PAGE and Western blot showed positive bands, indicating GST-Aβ 42 The fusion protein has been expressed in expression bacteria.
[0045] Induced condition optimization. Constructed pGEX-4T-1 / Aβ 42 / BL 21 expression strains were cultured at 37°C or 25°C, inoculated with fresh bacteria at a ratio of 1:100, and cultured with s...
Embodiment 3
[0049] Example 3: Aβ 42 Protein digestion and identification
[0050] (8) Aβ 42 Protease digestion and purification: purified GST-Aβ 42 The fusion protein was treated with thrombin, 10U / mg fusion protein, reacted in pH7.4, 50mM PBS phosphate buffer system at 23°C for 16 hours, and then passed through Glutathione Sepharose 4B affinity column to remove enzyme-cleaved GST protein, benzene Formamidine column removes thrombin to obtain pure Aβ 42 protein;
[0051] (9) Aβ 42 Western blotting identification of protein: the above-mentioned Aβ obtained by enzyme digestion and double affinity purification 42 Anti-Aβ 42 Antibody and anti-GST antibody were identified by Western blotting, and the results showed that the obtained protein could only interact with anti-Aβ 42 Antibody reaction, but not with anti-GST antibody, confirms that the protein is Aβ 42 protein;
[0052] (10) Aβ 42 Protein aggregation analysis: using the fluorescent substance Thioflavin-T that can specifically...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com