Dihydroxyl-terminated polydimethylsiloxane and preparation thereof
A technology of dihydroxyalkyl polydimethylsiloxane and epoxyalkyl polydimethylsiloxane, which is applied in the field of synthesizing single-end dihydroxylalkyl polydimethylsiloxane and can solve the problem of single-end dihydroxylalkyl polydimethylsiloxane The industrialization cost of polydimethylsiloxane is increased, unsaturated compounds are not easy to obtain, and the total reaction time is longer, etc., to achieve the effect of easy separation and purification, shorter reaction time, and fewer synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
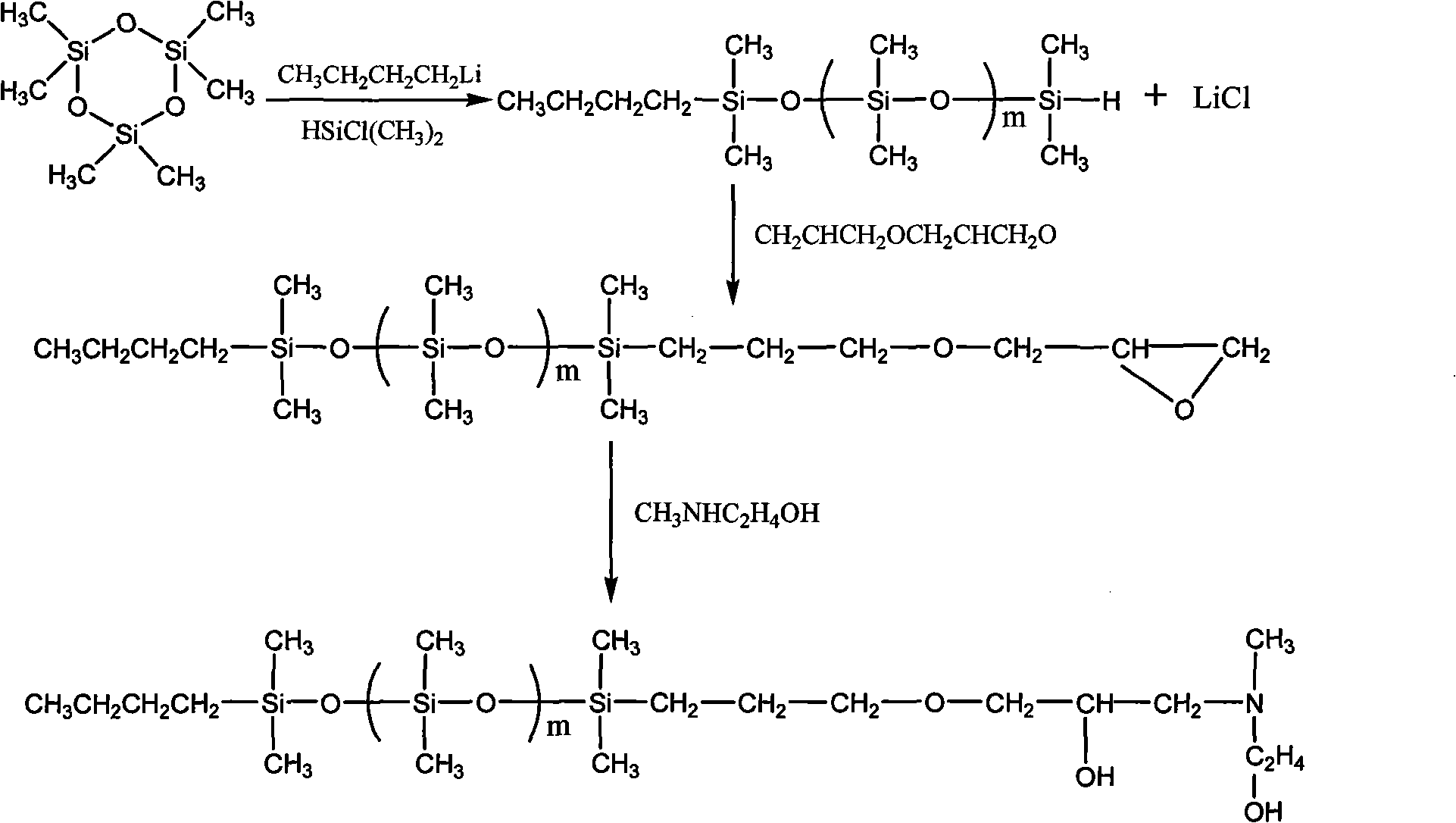
[0020] Anionic ring-opening polymerization reaction: Add D 3 (19.52g) of benzene solution and 0.014mol n-butyllithium, stirred at room temperature for 0.5h, then added 60ml of tetrahydrofuran, stirred at 25°C, reacted for 8h, added end-capping agent dimethylchlorosilane, after the end, filtered off The generated lithium chloride was distilled under reduced pressure to remove low boilers, and cooled to obtain 20.68 g of a colorless and transparent liquid single-ended hydrogen polydimethylsiloxane, with a yield of 98%.
[0021] Hydrosilylation reaction: In a 100ml four-neck flask, add a toluene solution of allyl glycidyl ether (2.78g) and an appropriate amount of catalyst (isopropanol solution of chloroplatinic acid), and after passing nitrogen gas for 0.5h, continue Ventilate and heat the reaction system to raise the temperature. After it is stabilized to 100°C, stir, add single-ended hydrogen polydimethylsiloxane (7.33g) dropwise, react for 8h, cool, remove low boilers by dist...
Embodiment 2
[0024] The ethanol in the epoxy ring-opening reaction of Example 1 was changed to methanol, and the reaction temperature was changed to 65° C., and other reaction conditions were as described in Example 1 to obtain the target compound single-ended dihydroxyalkyl polydimethylsiloxane, Yield 95%.
Embodiment 3
[0026] The ethanol in the epoxy ring-opening reaction in Example 1 was changed to n-propanol, the reaction temperature was changed to 90°C, and other reaction conditions were as described in Example 1 to obtain the target compound single-ended dihydroxyalkyl polydimethylsiloxane Alkanes, yield 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com