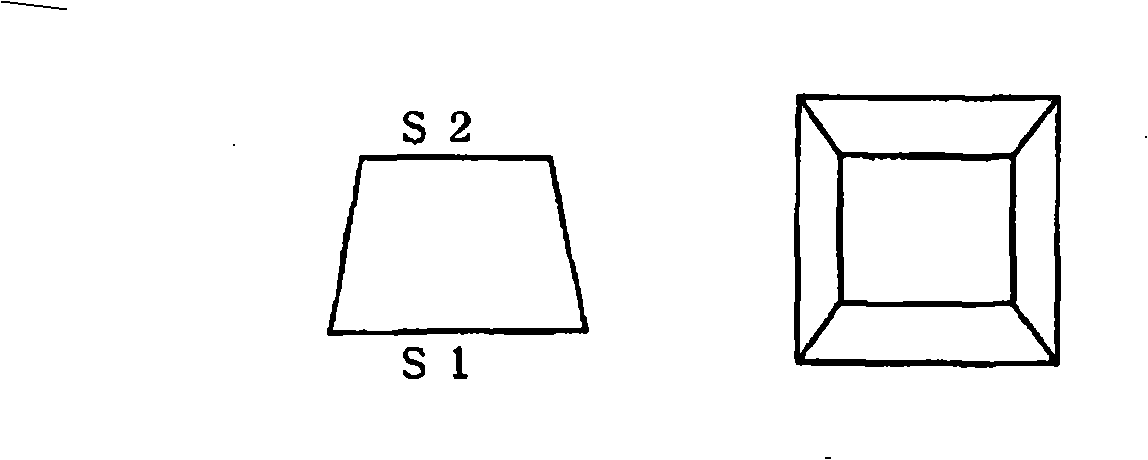
Active energy ray-curable composition
A curable composition and active energy ray technology, which is applied in the field of active energy ray curable compositions, can solve problems such as insufficient sensitivity, inability to obtain resolution, and residual coating film
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
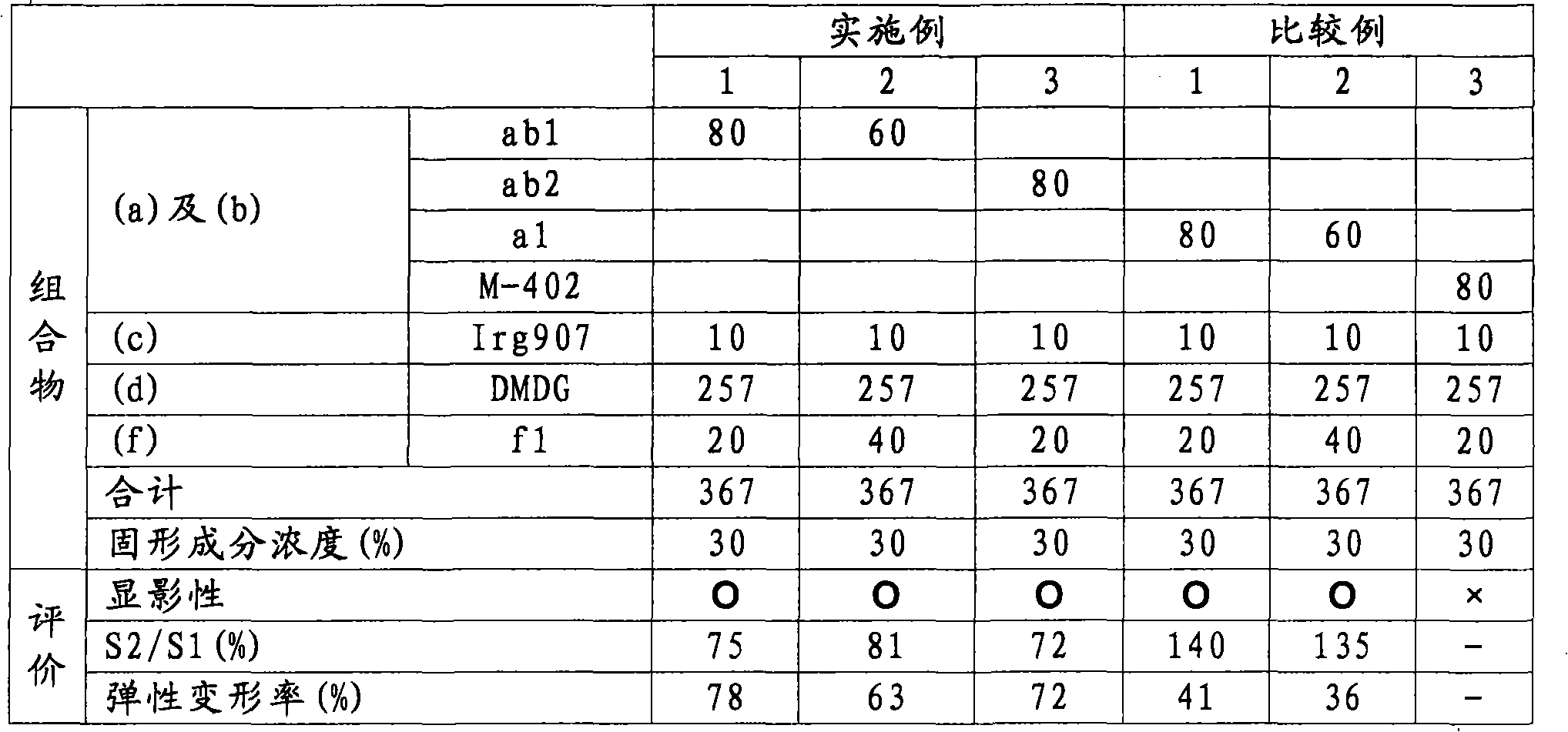
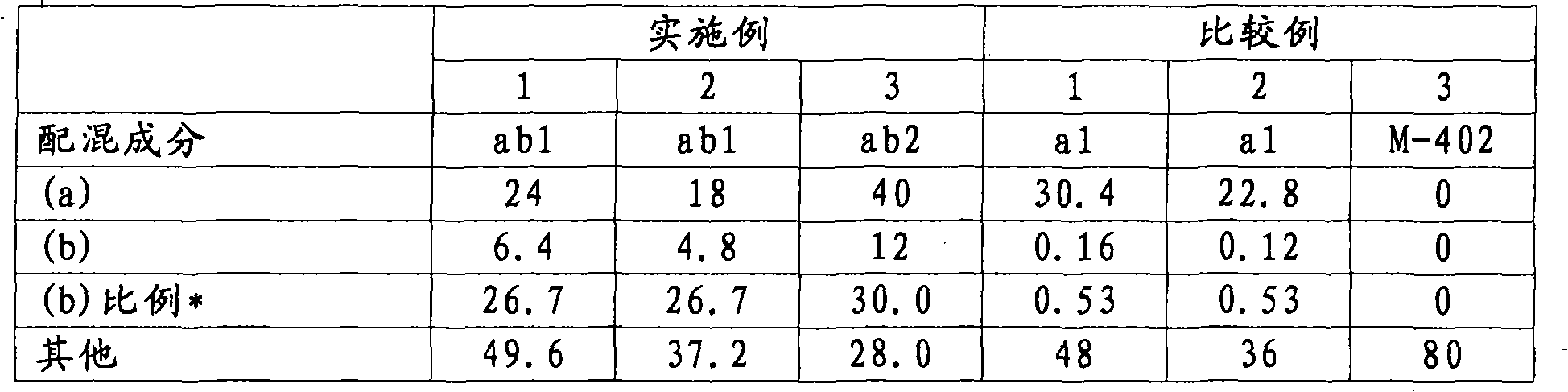
[0158] Hereinafter, the present invention will be described more specifically with reference to Examples and Comparative Examples.
[0159] In addition, the following "part" means a mass part, and "%" means a mass %.
manufacture example 1
[0161] (1) Esterification reaction
[0162] As raw materials, 400 g (1.58 mol) of dipentaerythritol and 821 g (11.4 mol) of acrylic acid were used. As a solvent, 775 g of toluene was used, and 2.2 g of hydroquinone monomethyl ether (hereinafter referred to as "MQ") as a polymerization inhibitor were added to the above-mentioned raw material, 17 g of sulfuric acid as a catalyst, and esterification was carried out at 90°C. 6 hours.
[0163] (2) Neutralization treatment
[0164] After the above reaction was completed, 1690 g of toluene was added, the reaction solution was filtered with filter paper, and then washed with 875 g of distilled water, and 800 g of 10% NaOH aqueous solution was added to the washed reaction solution, and neutralized at room temperature for 1 hour. .
[0165] (3) Washing treatment
[0166] After the above-mentioned neutralization treatment, the organic layer and the water layer were separated, and the water layer was removed. In order to further remov...
manufacture example 2
[0179] (1) Esterification reaction
[0180] Except that the raw material acrylic acid was set to 683 g (9.48 mol), esterification reaction was carried out in accordance with Production Example 1 in all cases.
[0181] (2) Neutralization treatment
[0182] All were neutralized according to Production Example 1.
[0183] (3) Washing treatment
[0184] All were washed with water according to Production Example 1. An acrylate (hydroxyl value: 72 mgKOH / g) was obtained.
[0185] (4) acid modification
[0186] In a glass flask, 250 g of the acrylate obtained in (3) was charged, 32 g of succinic anhydride and 0.13 g of hydroquinone monomethyl ether were added, and the temperature was raised to 85°C. Among them, after 1.3 g of catalyst triethylamine was charged, reaction was performed for 4 hours. The reaction was performed under a mixed atmosphere of air / nitrogen to obtain a compound having an acid value of 68 mgKOH / g (hereinafter referred to as "ab2"). As a result of analyzing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com