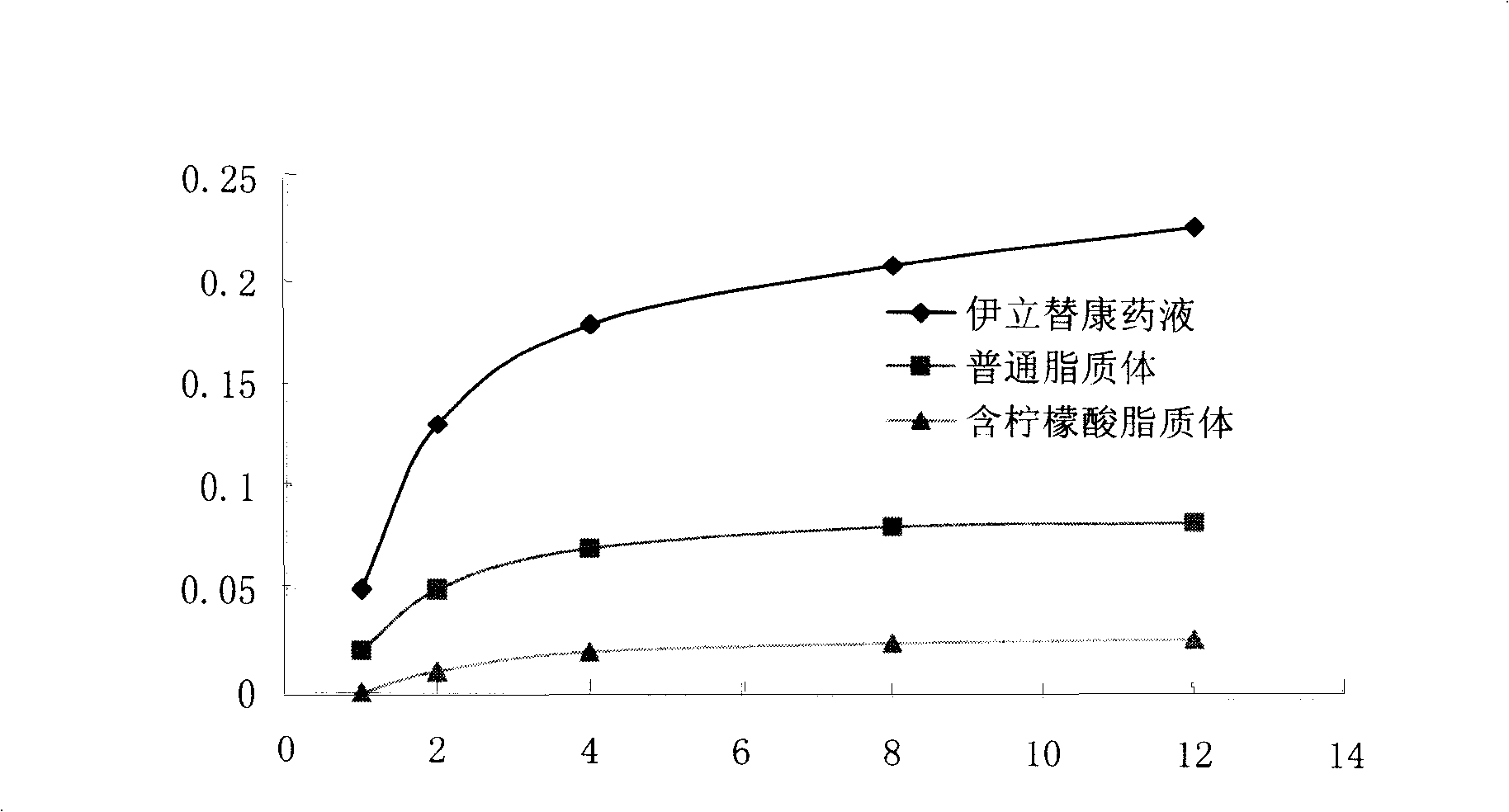
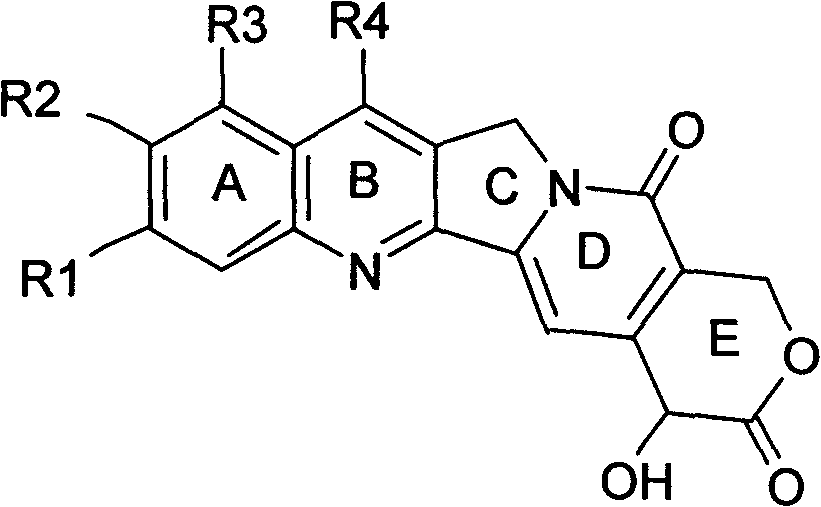
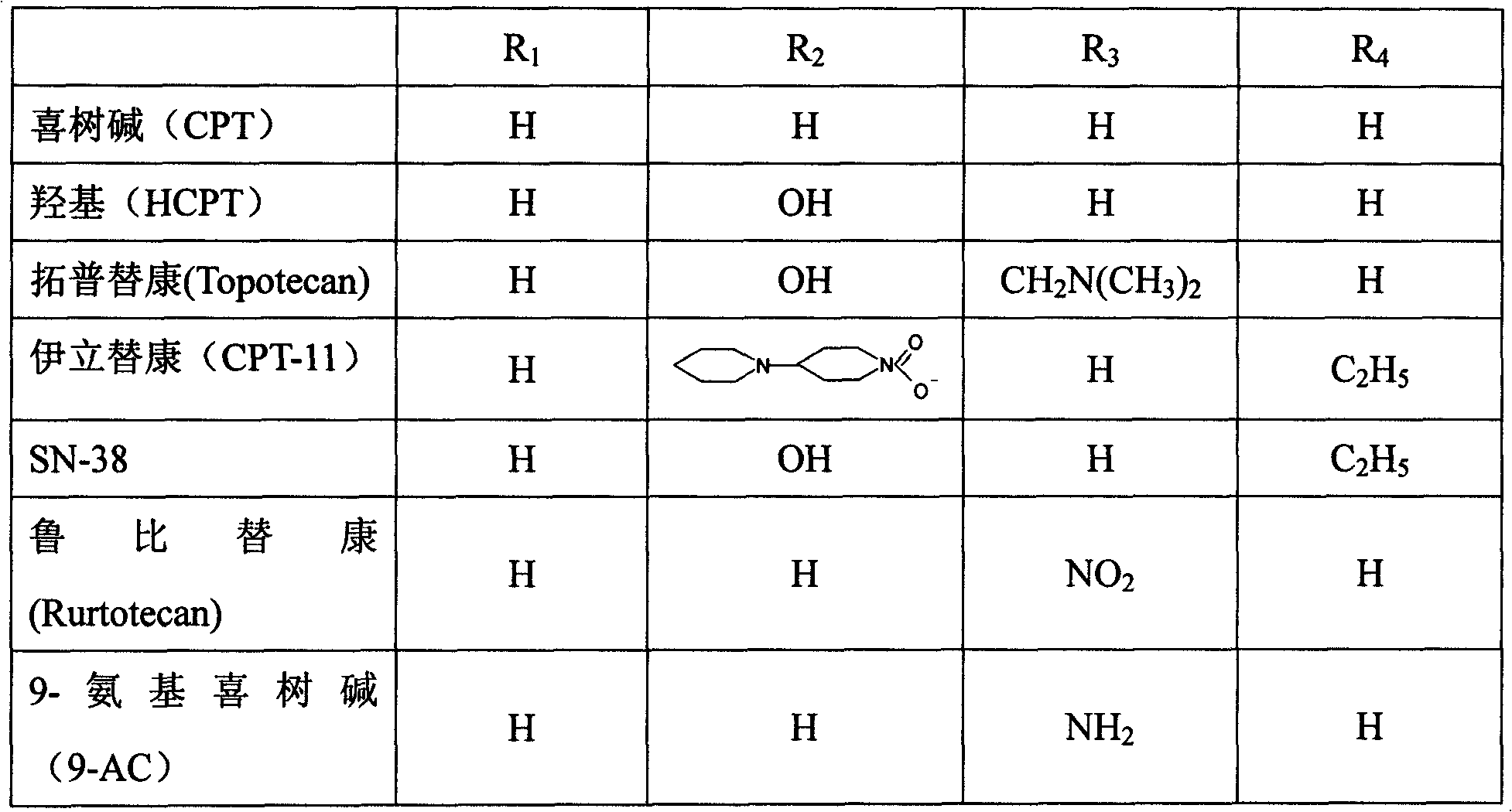
Stable camptothecine liposome composition
A technology of liposome composition and camptothecin, which is applied in the directions of drug combination, liposome delivery, anti-tumor drugs, etc., can solve the problems of lactone ring-opening reaction, toxic and side effects, and curative effect reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Soy Lecithin: 1g
[0040] Cholesterol 0.1g
[0041] Ascorbic Acid 50mg
[0042] Vitamin E 10mg
[0043] Irinotecan Hydrochloride 50mg
[0044] Trehalose 2.0g
[0045] Weigh 50 mg of irinotecan hydrochloride, 1.0 g of soybean lecithin, 0.1 g of cholesterol, 50 mg of ascorbic acid, and 10 mg of vitamin E and dissolve them in 100 ml of ethanol, place the solution in a round-bottomed flask, and use a rotary evaporator on a constant temperature water bath at 40°C Ethanol was evaporated under the condition of 50rpm and reduced pressure, so that membrane materials such as phospholipids formed a uniform lipid membrane at the bottom of the flask, placed in a vacuum desiccator, and placed at room temperature for 24 hours for later use. After taking it out, add 30ml of pH7.4 phosphate buffer solution, and then wash the membrane by rotating the above-mentioned round-bottom flask at 37°C with a rotary evaporator until the lipid membrane is hydrated and becomes a milky white lipo...
Embodiment 2
[0047] Hydrogenated phospholipids: 0.6g
[0048] Cholesterol 0.1g
[0049] Maleic acid 100mg
[0050] Vitamin E 15mg
[0051] 10-Hydroxycamptothecin 20mg
[0052] Glucose 1.5g
[0053] Dissolve 20mg of 10-hydroxycamptothecin and 100mg of maleic acid in 20ml of water for injection, and dissolve 0.6g of hydrogenated phospholipids, vitamin E and 0.1g of cholesterol in 100ml of ether, slowly inject the ether phase into the continuously stirring water phase, Water-bath ultrasonication until the emulsification is uniform, put it into a round-bottomed flask with a ground mouth, and evaporate the ether with a rotary evaporator on a 40°C constant temperature water bath at 100rpm and under reduced pressure. After the micelles are formed, add 30ml pH7. 4 Phosphate buffer solution, and then rotate the above-mentioned round-bottomed flask with a rotary evaporator at 37°C until the micelles are evenly dispersed in the hydration medium to obtain a milky white liposome suspension. 1.5 g ...
Embodiment 3
[0055] Lecithin: 1.2g
[0056] PEG-DSPE0.8g
[0057] Cholesterol 0.3g
[0058] Malic acid 50mg
[0059] Topotecan 50mg
[0060] Lactose 100mg
[0061] Sucrose 2.0g
[0062] Inject nitrogen into 10ml water for injection for 10min, add 5mg topotecan, 50mg malic acid, 0.8g PEG-DSPE, 1.2g lecithin and 0.3g cholesterol after nitrogen flushing, and use a high-speed stirrer to mix the mixture at 65°C Disperse for 10 minutes, then emulsify the pre-dispersion 3 times in a high-pressure homogenizer (7500psi, 65°C), cool the liposomes to room temperature, add 100mg lactose, 2.0g sucrose, dissolve in the liposome suspension, After aseptic filtration with a 0.22 μm microporous membrane, it is divided into vials, freeze-dried, and obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com