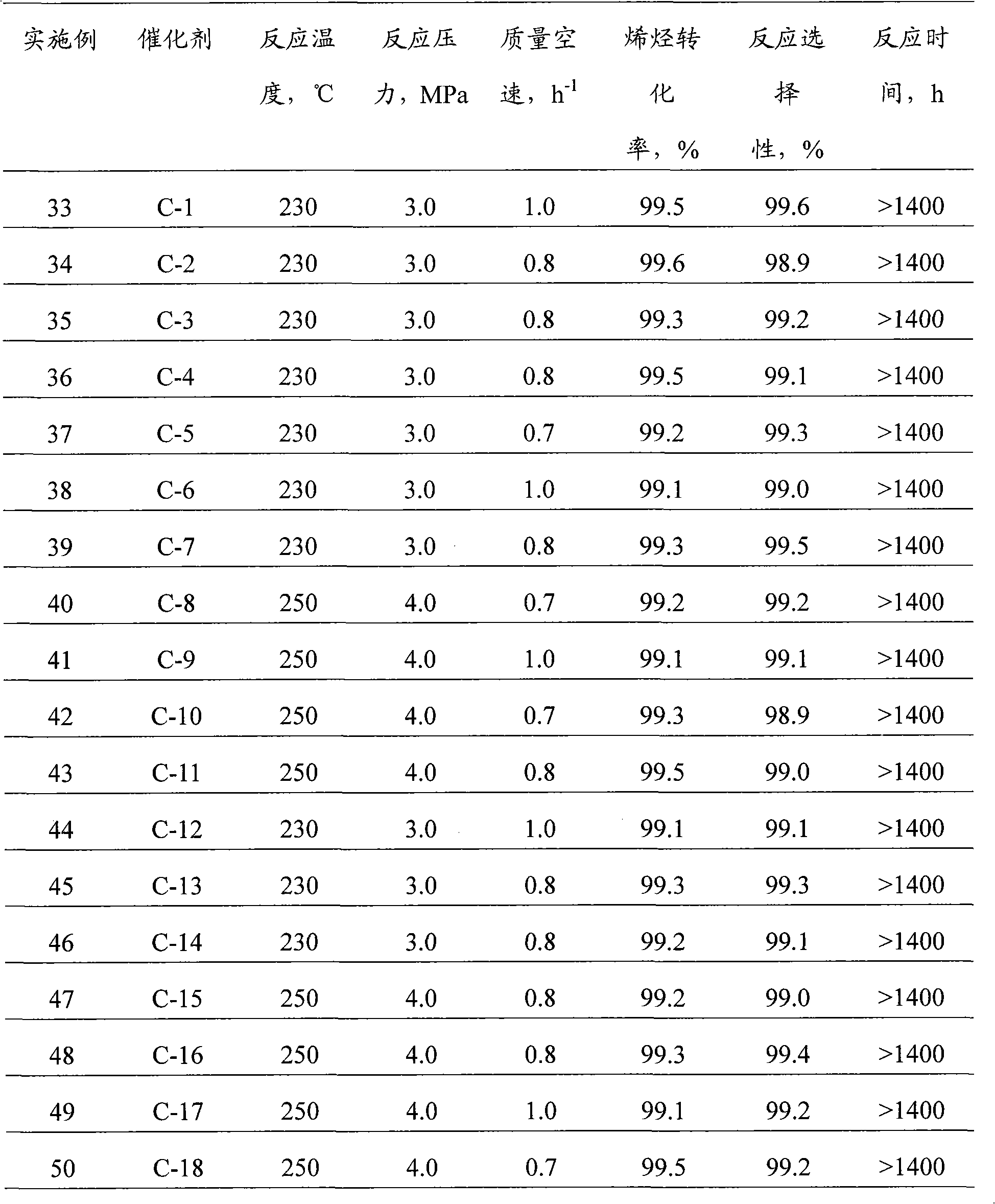Method for synthesizing linear alkylbenzene
A technology of straight-chain alkyl benzene and synthesis method, applied in chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve problems such as unfavorable industrial production, high energy consumption, etc., and avoid frequent switching operations of reaction and regeneration. , high olefin conversion, high olefin conversion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
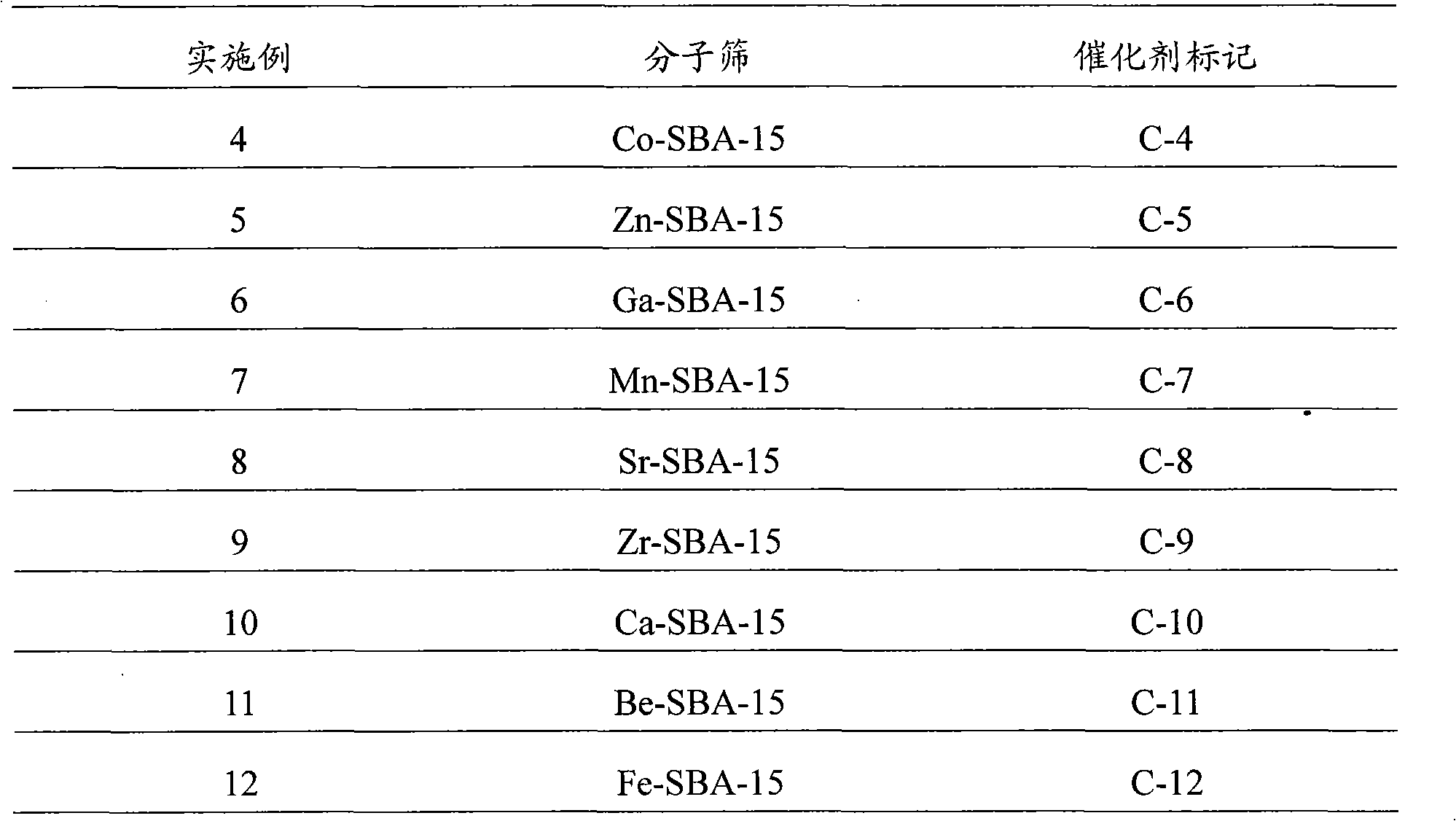
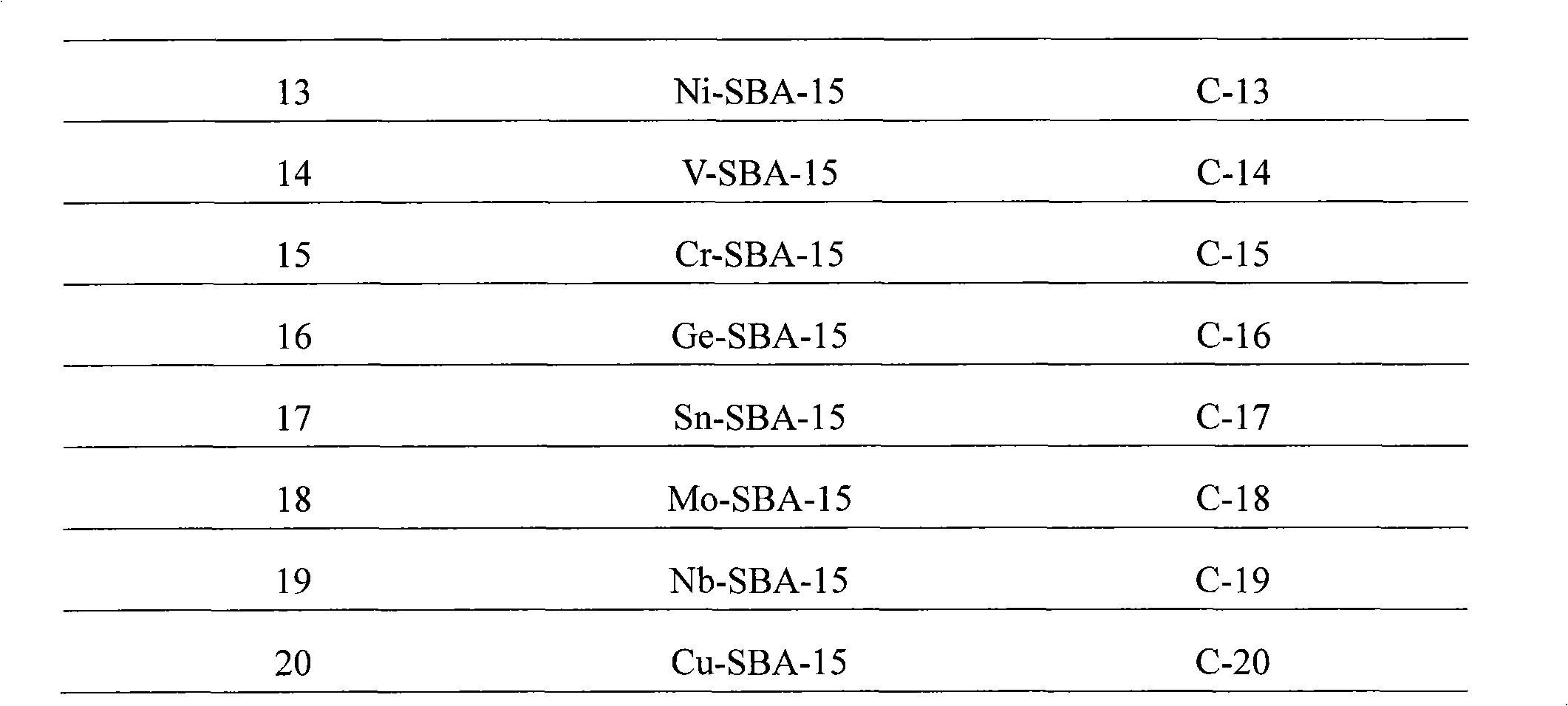
Embodiment 1
[0036] Embodiment 1: the synthesis of Al-SBA-15 type molecular sieve catalyst
[0037] According to the molar ratio of raw materials P123: Al 2 o 3 : SiO 2 :HCl:H 2 O is calculated as 1:3.1:62.5:300:10000, weigh 20 grams of triblock polymer P123, mix with calculated amount of distilled water and hydrochloric acid, stir and mix at 40°C for 1 hour, add calculated amount of alumina monohydrate , continue to stir and mix for 1h; then, slowly add the calculated amount of tetraethyl orthosilicate under stirring conditions, and continue to stir for 5h at 40°C; crystallize at 100°C for 48 hours, then filter, wash, and dry. Finally, the temperature was programmed (at 100°C, 200°C, 300°C, and 400°C for 1h respectively) to 550°C for 5h, and the template agent was removed to obtain the Al-SBA-15 molecular sieve catalyst, which was designated as C-1 catalyst.
Embodiment 2
[0038] Embodiment 2: the synthesis of W-SBA-15 type molecular sieve catalyst
[0039] According to the molar ratio of raw materials P123: EtOH: WO 3 : SiO 2 :HCl:H 2 O is calculated as 1:200:6.25:62.5:350:13000, weigh 20 grams of triblock polymer P123, mix with calculated amount of distilled water, ethanol and hydrochloric acid, stir and mix at 40°C for 1 hour, add calculated amount of Tungstic acid, continue to stir and mix for 1 hour; then, slowly add the calculated amount of ethyl orthosilicate under stirring conditions, and continue to stir for 5 hours at 40°C; crystallize at 100°C for 48 hours, followed by filtration, washing, After drying, the temperature was programmed to rise to 550° C. for 5 h to remove the templating agent to obtain a W-SBA-15 molecular sieve catalyst, which was designated as C-2 catalyst.
Embodiment 3
[0040] Embodiment 3: the synthesis of Ti-SBA-15 type molecular sieve catalyst
[0041] According to the molar ratio of raw materials P123:TiO 2 : SiO 2 :HCl:H 2 O is calculated as 1:6.25:62.5:350:13000, weigh 20 grams of triblock polymer P123, mix with the calculated amount of distilled water and hydrochloric acid, stir and mix at 40°C for 1 hour; then, slowly add Add the calculated amount of ethyl orthosilicate, and add the calculated amount of butyl titanate (Jinshan Xingta Meixing Chemical Factory) at the same time, continue to stir at 40°C for 5h; crystallize at 100°C for 48 hours, and then filter , washed, dried, and finally programmed to heat up to 550 ° C for 5 hours to remove the template agent to obtain a Ti-SBA-15 molecular sieve catalyst, which is designated as C-3 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com