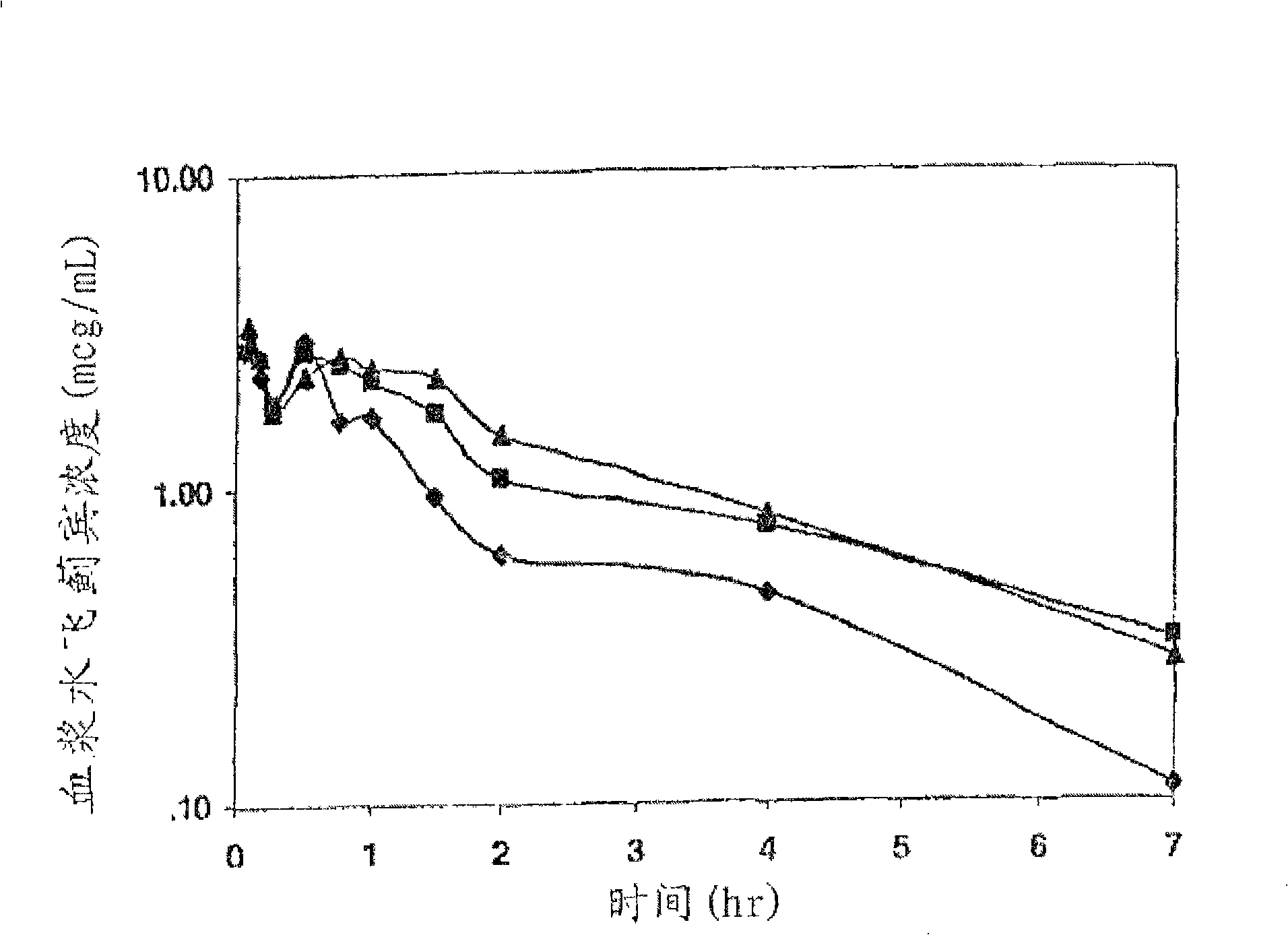
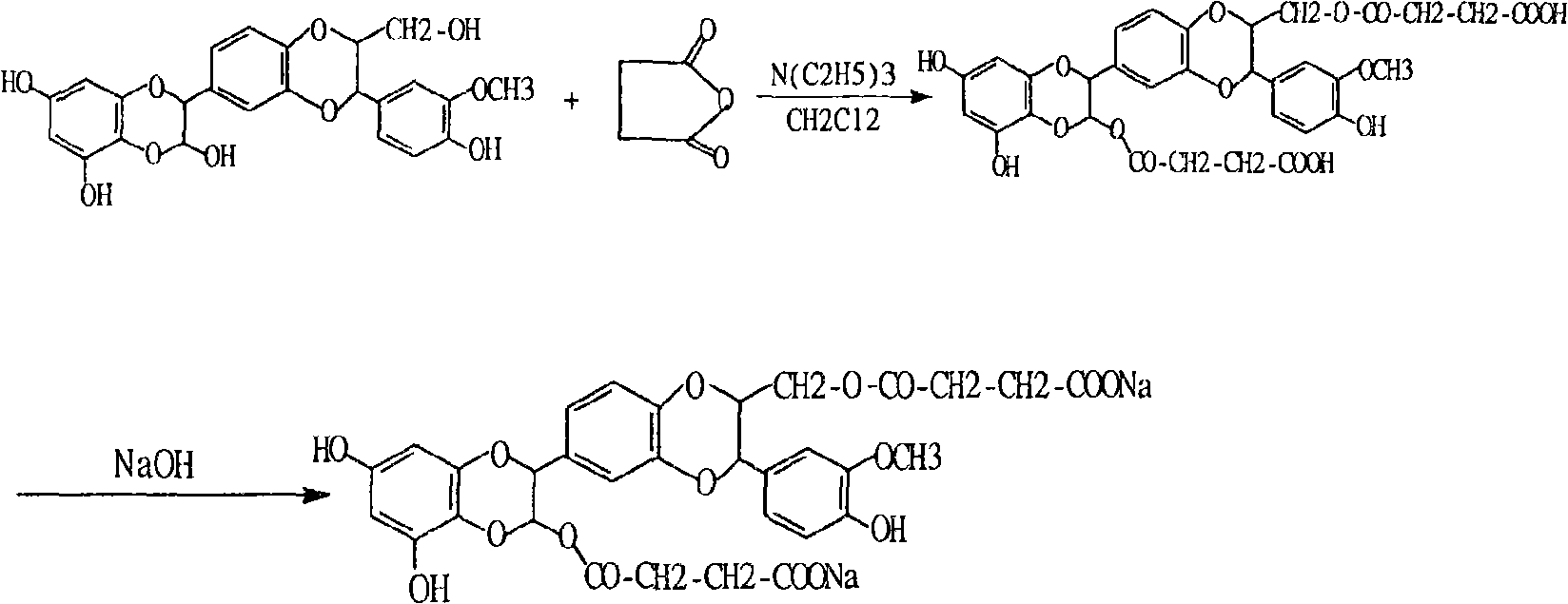
Preparation and use of silybum marianum di-partial succinate and salt thereof
A technology of dimetasuccinate and silibinin, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, and the digestive system, and can solve complex extraction and washing operations, side reactions, and influence on product purity and quality, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Add 48.2 grams (0.1 moles) of silibinin, 30.3 grams (0.3 moles) of triethylamine, and 40 grams (0.4 moles) of succinic anhydride to a garden flask with a drying system containing 500 milliliters of dichloromethane , stirring at room temperature, G254 thin layer detection tracking (developing agent is chloroform: methanol: ethyl acetate: water = 15:6:15:1, the reaction is completed in about 2 hours, the organic layer is washed with 10% hydrochloric acid, and then washed with water, The organic layer was dried over anhydrous sodium sulfate, and was concentrated to obtain 61.4 grams of white solid (yield was 90%).60 grams of silybin succinate methyl ester of gained were dissolved in 100 milliliters of methanol, at room temperature and hydrogen Sodium oxide solution was reacted to obtain 60 grams of alcohol-insoluble sodium salt with a yield of 94%.
Embodiment 2
[0012] Add 48.2 grams (0.1 moles) of silibinin, 30.3 grams (0.3 moles) of triethylamine, and 40 grams (0.4 moles) of succinic anhydride to a round-bottomed flask with a drying system filled with chloroform, and stir at room temperature For about 2.5 hours, wash the organic layer with 10% hydrochloric acid, wash with water, dry the organic layer with anhydrous sodium sulfate, and concentrate to obtain 60.5 grams of white solid (yield 88.6%)
Embodiment 3
[0014] 48.2 grams (0.1 moles) of silibinin, 30.3 grams (0.3 moles) of triethylamine, and 40 grams (0.4 moles) of succinic anhydride were added to a garden flask with a drying system filled with ethyl acetate, at room temperature Stir for about 4 hours, wash the organic layer with 10% hydrochloric acid, wash with water, dry the organic layer with anhydrous sodium sulfate, and concentrate to obtain 58.3 g of white solid (yield: 85.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com