Matrix metalloproteinase targeted recombined human tumor necrosis factor and preparation thereof
A tumor necrosis factor and matrix metal technology, which is applied in recombinant DNA technology, hybrid peptides, and introduction of foreign genetic material using vectors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
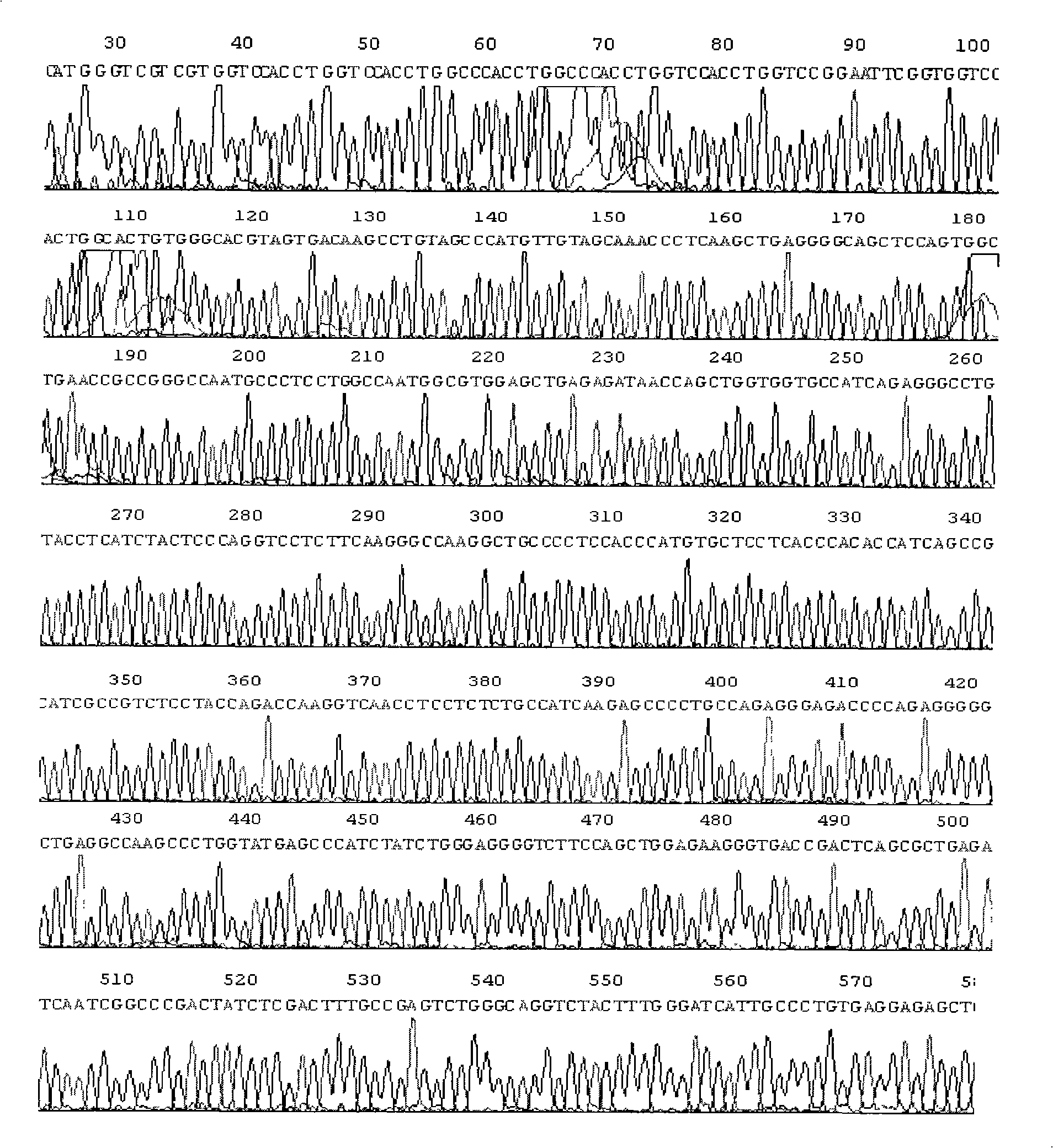
[0065] Example 1. Cloning of Human Tumor Necrosis Factor-α cDNA (pGEM-hTNF-α)
[0066] 1.PCR primers and their sequences (amplification product 650bp)
[0067] Sense primer: 5′CG GAATTC CTGCTGCACTTTGGAGTGATCG 3′
[0068] The underline is the EcoR I restriction site
[0069] Anti-sense primer: 5′GC GAGCTCG TTTGAGCCAGAAGAGGTTGAGGG 3′
[0070] The underline is the Sac I restriction site
[0071] 2. RT-PCR
[0072] Mononuclear cells were isolated from human venous blood according to conventional methods, and after being stimulated with LPS (100ng / ml) for 3 hours, total cellular RNA was extracted; RT-PCR reaction was carried out using this as a template, with a total reaction volume of 50 μl. The reaction conditions were: denaturation at 94°C for 1 min, annealing at 65°C for 1 min, extension at 72°C for 1 min, and a total of 30 cycles.
[0073] 3. Clone
[0074] After the PCR product was purified, it was digested with EcoRI and SacI, ...
Embodiment 2
[0075] Example 2. Construction of the collagen coding sequence recombinant plasmid pGEM-collagen
[0076] 1. Collagen repeat oligonucleotide sequence
[0077] The collagen sequence is characterized by an amino acid sequence of (Gly-X-Y)n, where X and Y represent any amino acid (proline is the best) except Gly (glycine), and n is any positive integer (representing the number of repetitions). Therefore, there are many sequences that meet this characteristic, for example: positive strand (65nt):
[0078] 5'CATGGGTCGTCGTGGTCCACCTGGTCCACCTGGCCCACCTGGCCCACCTGGTCCACCTGGTCCGG 3' negative strand (65nt):
[0079] 5′AATTCCGGACCAGGTGGACCAGGTGGGCCAGGTGGGCCAGGTGGACCAGGTGGACCACGACGACC 3′
[0080] Annealing to form double strands (Nco I enzyme site and EcoR I enzyme site with two separate bands in the double strand)
[0081] 2. Cloning
[0082] The double strand formed by annealing was directional ligated to pGEM-T Easy vector (NcoI and EcoRI double enzyme digestion), and transformed into...
Embodiment 3
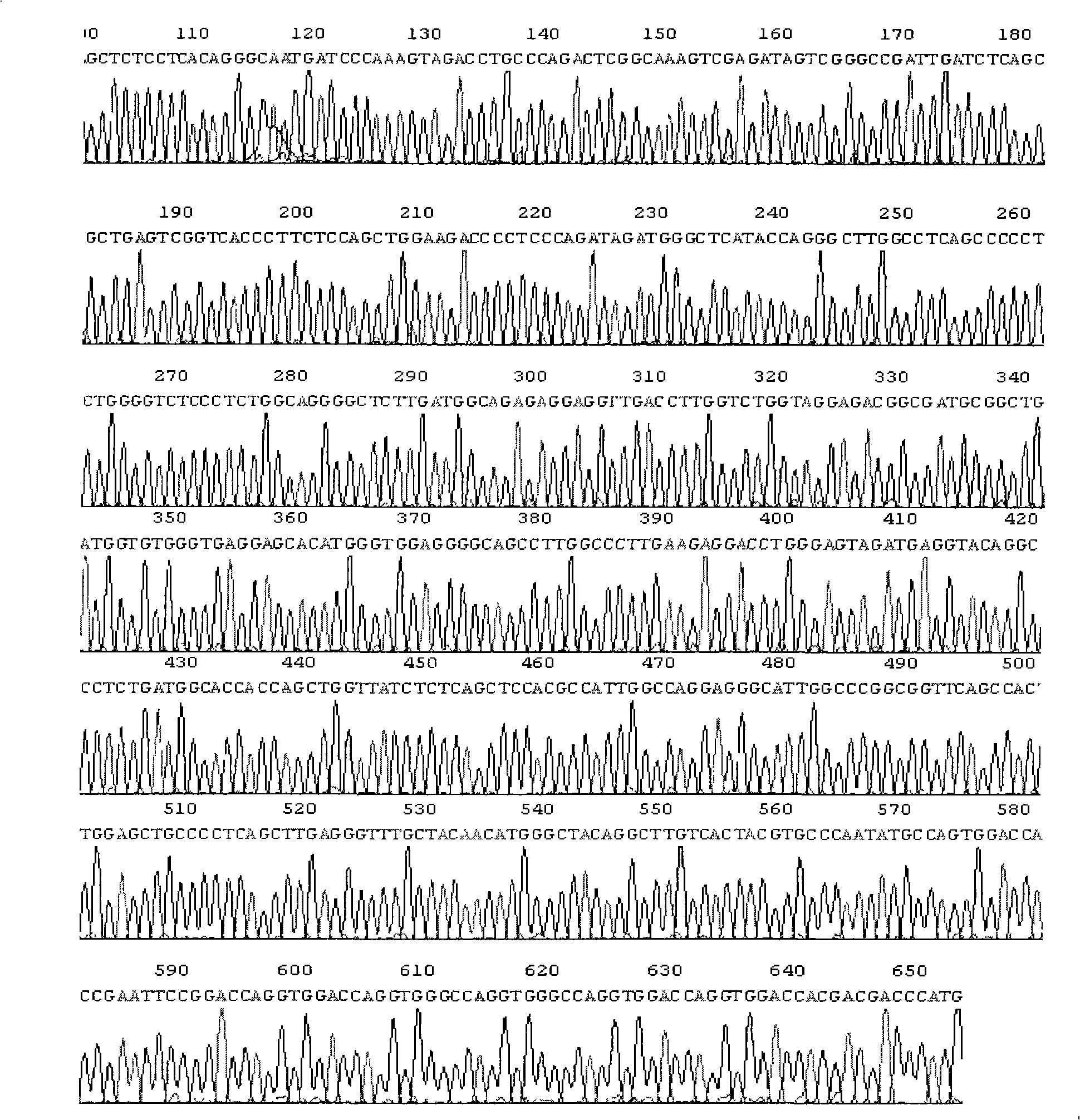
[0083] Embodiment 3. Construction of the purpose fusion protein gene plasmid (pGEM-collagen-MMPnm-hTNF)
[0084] 1.PCR primers and their sequences
[0085] Since there are more than ten kinds of MMPs that have been discovered, they can be divided into five categories. Therefore, for each type of MMP, 2-4 corresponding suitable substrate sequences (realized by primer design) are designed and corresponding recombinant plasmids are constructed. For example:
[0086] Anti-sense primer (corresponding to the hTNF-α full-length cDNA stop codon and the following 3 base positions):
[0087] 5′GC GAGCTC TCCTCACAGGGCAATGATCCCAAAG 3′
[0088] The underline is the SacI restriction site
[0089] Sense primers (corresponding to positions 410-431 of hTNF-α full-length cDNA):
[0090] 5’ CGGAATTC GGTGGT………AGTGACAAGCCTGTAGCCCATGTTG 3’
[0091] Wherein, the underlined place is the restriction site of EcoR I, ... represents a suitable substrate sequence (there are many kinds) of a class of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com