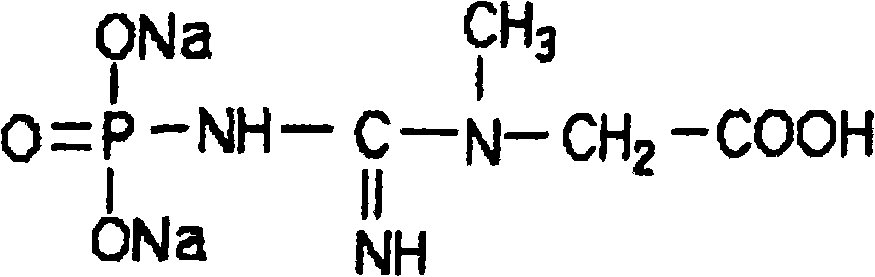
Preparation technique for sodium phosphocreatine freeze-dried injection
A technology of sodium phosphate phosphate and freeze-dried powder injection, which can be used in freeze-dried delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Problems such as poor clarity of sodium powder injection, to achieve the effect of easy operation and good clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
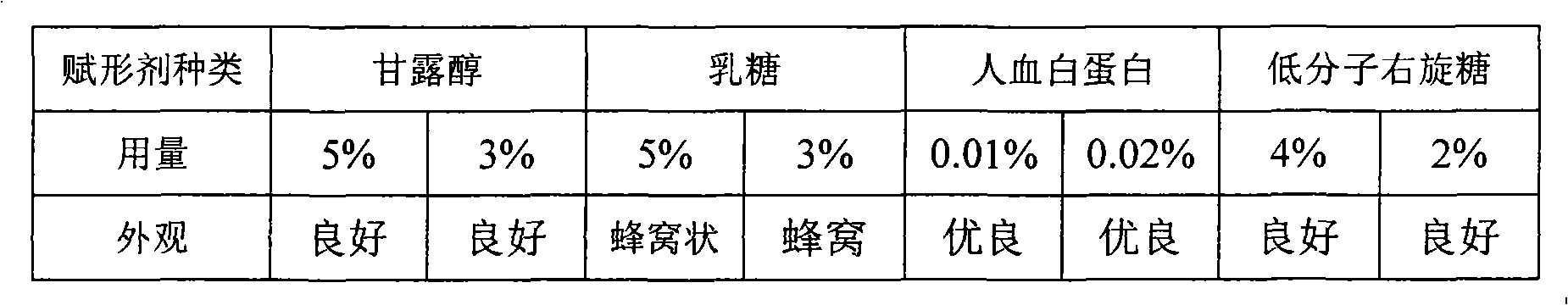
[0022] Excipient Screening Determination
[0023]
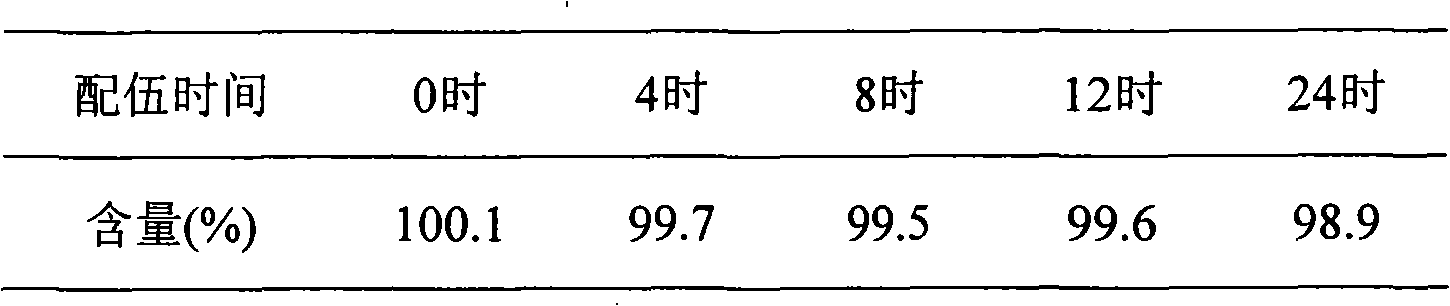
[0024] The results show that creatine phosphate sodium combined with human serum albumin has a better appearance, mannitol and low molecular weight dextrose also have a good shape, and the reconstitution time is less than 30 seconds, so it is determined that human serum albumin, mannitol and low molecular weight dextrose have a good appearance. Molecular dextrose is the excipient of this product.
[0025] (1) Freeze-dried powder injection prescription:
[0026] Sodium creatine phosphate (calculated as anhydrous substance) 500g
[0027] Human serum albumin (1% concentration: D=1.0077g / ml) 20g
[0028] Water for injection 1500g
[0029]
[0030] Makes 1000 bottles
[0031] (2) Preparation process:
[0032] Weigh 500g of creatine phosphate sodium (based on anhydrous matter), add it to 1500g of water for injection, add 20g of human serum albumin (1% concentration: D=1.007...
Embodiment 2
[0034] (1) Freeze-dried powder injection prescription:
[0035] Sodium creatine phosphate (calculated as anhydrous matter) 1000g
[0036] Human serum albumin (1% concentration: D=1.0077g / ml) 45g
[0037] Water for injection 3000g
[0038]
[0039] Makes 1000 bottles
[0040] (2) Preparation process:
[0041] Weigh 1000g of creatine phosphate sodium, add it to 3000g of water for injection, add human serum albumin, 1% concentration: D=1.0077g / ml, 45g, stir and dissolve evenly, add 2.4g of activated carbon for needles, stir for 40 minutes, Use titanium alloy rods to filter decarburization, control the pH value between 8.5 and 9.0, add water for injection to a total volume weight of 4047.4g, filter and sterilize with a 0.22μm microporous membrane, divide the filtrate into 1000 parts, and pack in In a 20ml sterile clean glass bottle, add butyl rubber stopper, keep the sublimation channel, put it in a freeze dryer, set the pre-f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com