Recombined nattokinase oral preparation, preparation method and application thereof
A technology of nattokinase and oral preparations, applied in the field of pharmacy, can solve the problem that there is no research report on oral preparations of recombinant nattokinase, and achieve the effects of uniform appearance, no adhesion and high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Cloning the full-length gene of nattokinase and constructing the expression plasmid of Bacillus subtilis
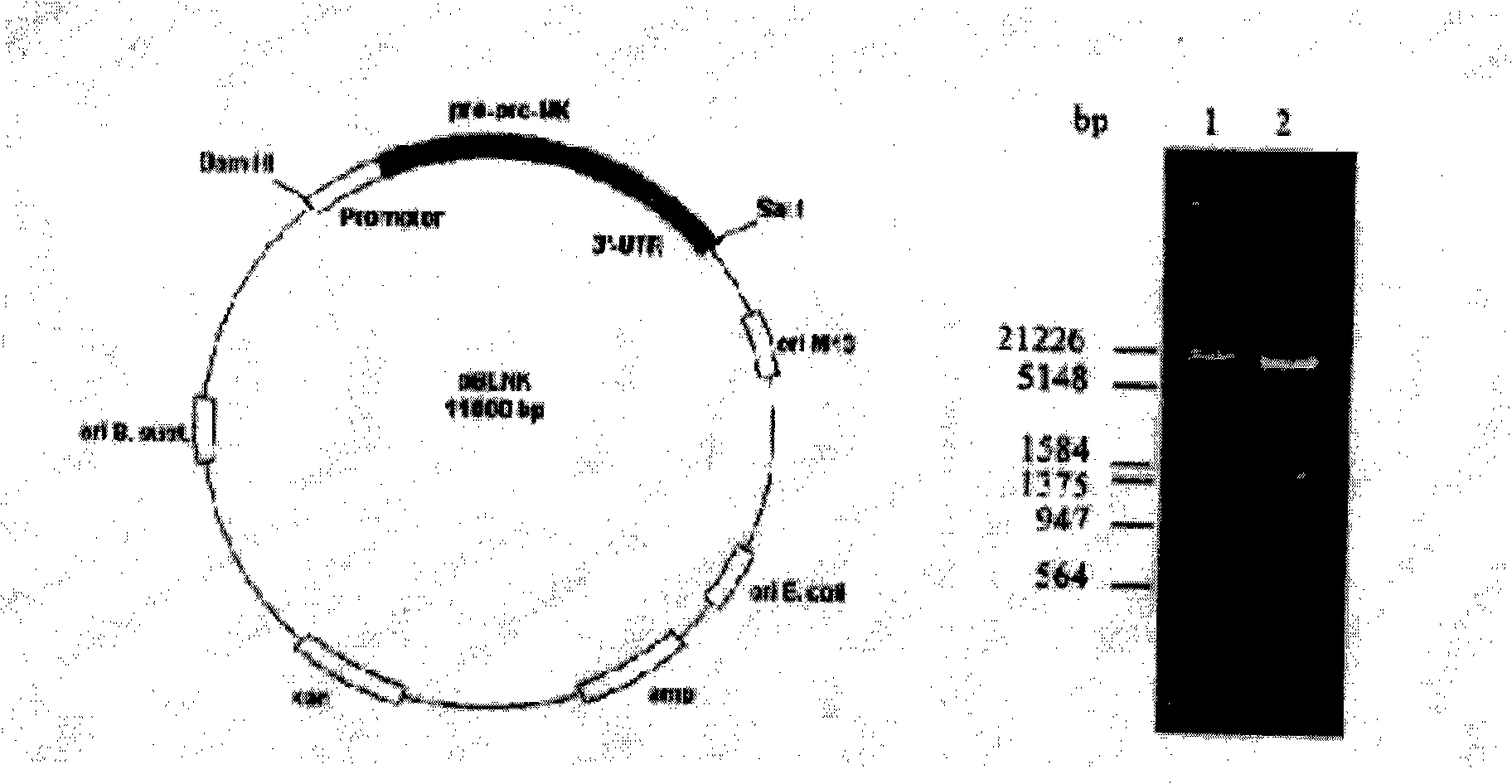
[0036] 1. Extract Bacillus natto genomic DNA (sequence 1), design cloning primers, and perform PCR with upstream primers 5'...GCGGGA TTC GTA TGA AAA TAG TTA...3' and downstream primers 5'...GTA GTC GACTCC GGT GCT TGT GAA...3' Amplify to obtain nattokinase gene and construct plasmid pBL NK.
[0037] 2. Expression of pBL NK in Bacillus subtilis expression system: Transform pBL NK plasmid into protease-deficient Bacillus subtilis by protoplast method, pick transformed colonies and inoculate them in 3ml LB culture medium containing 10μg kanamycin, shake at 34.5°C Shake for 15 hours, collect the culture solution for SDS-PAGE and activity analysis, and screen the seed engineering bacteria.
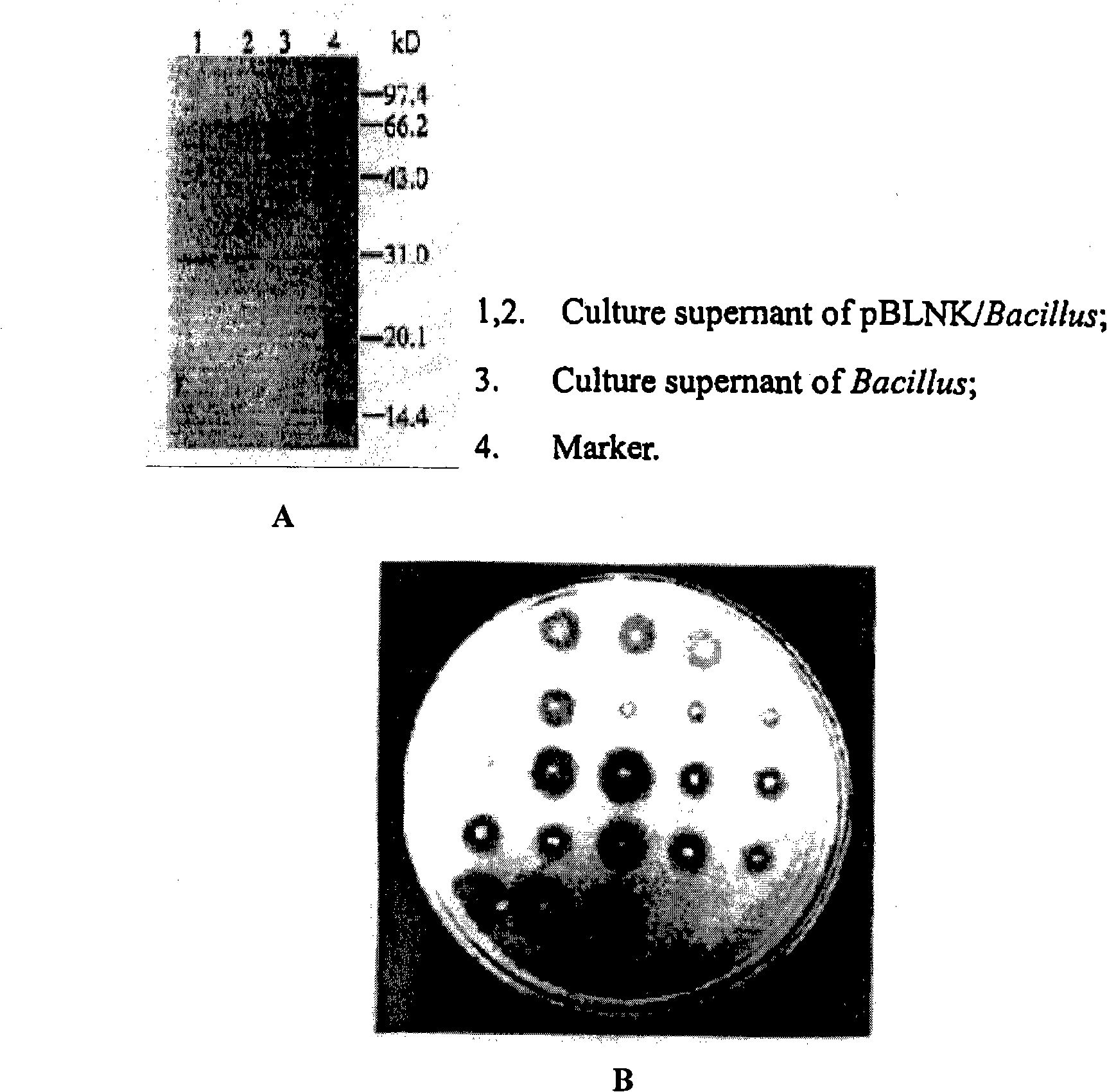
[0038]3. Purification of recombinant nattokinase: Pick freshly scratched monoclonal seed bacteria, inoculate in 3ml LB culture medium containing 10μg kanamycin, shake at ...
Embodiment 2
[0058] 1. Extract Bacillus natto genomic DNA, use upstream primer 5'...GCG GGA TTC GTA TGAAAA TAG TTA...3' and downstream primer 5'...GTA GTC GAC TCC GGT GCT TGTGAA...3' for PCR amplification to obtain nattokinase gene , construction of plasmid pBL NK
[0059] 2. Expression of pBL NK in Bacillus subtilis expression system: Transform pBL NK plasmid into protease-deficient Bacillus subtilis by protoplast method, pick transformed colonies and inoculate them in 3ml LB culture medium containing 10μg kanamycin, shake at 34.5°C Shake for 15 hours, collect the culture solution for SDS-PAGE and activity analysis, and screen the seed engineering bacteria.
[0060] 3. Purification of recombinant nattokinase: Pick freshly scratched monoclonal seed bacteria, inoculate in 3ml LB culture medium containing 10μg kanamycin, shake at 37°C for 12h, inoculate in 1L culture medium at 1:1000 Shake at 34.5°C for 15 hours, centrifuge at 8000rpm and collect the supernatant. 1L of the supernatant is ul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| critical point | aaaaa | aaaaa |
| critical point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com