Salicylic-g-chitosan oligosaccharide grafts and synthesizing process
A technology of chitosan oligosaccharide and salicylic acid, which is applied in the field of salicylic acid-g-chitosan oligosaccharide graft and its synthesis, can solve the problems of poor solubility, troublesome handling, limitation and the like, and achieves abundant administration means. , Easy post-processing, good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
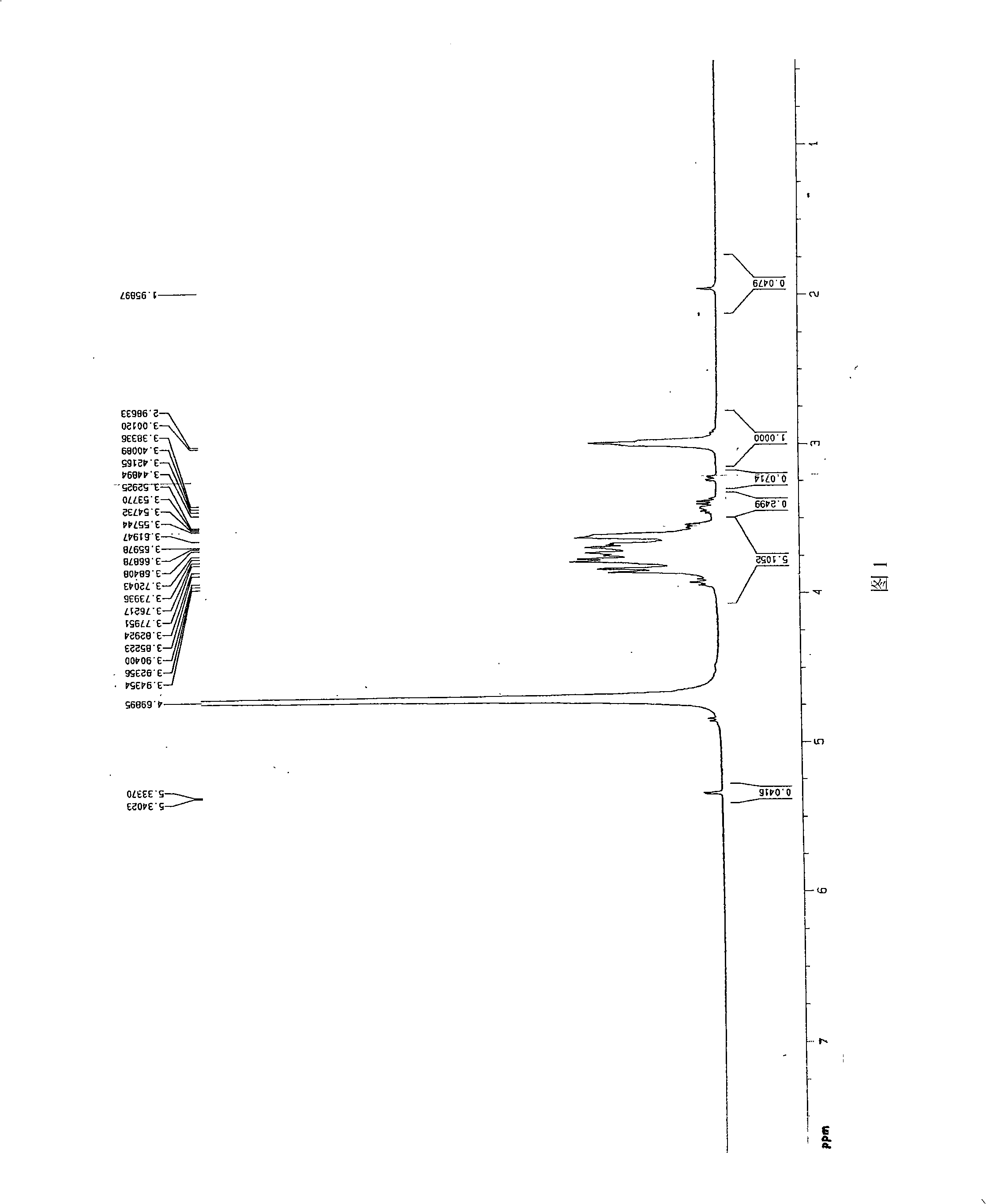
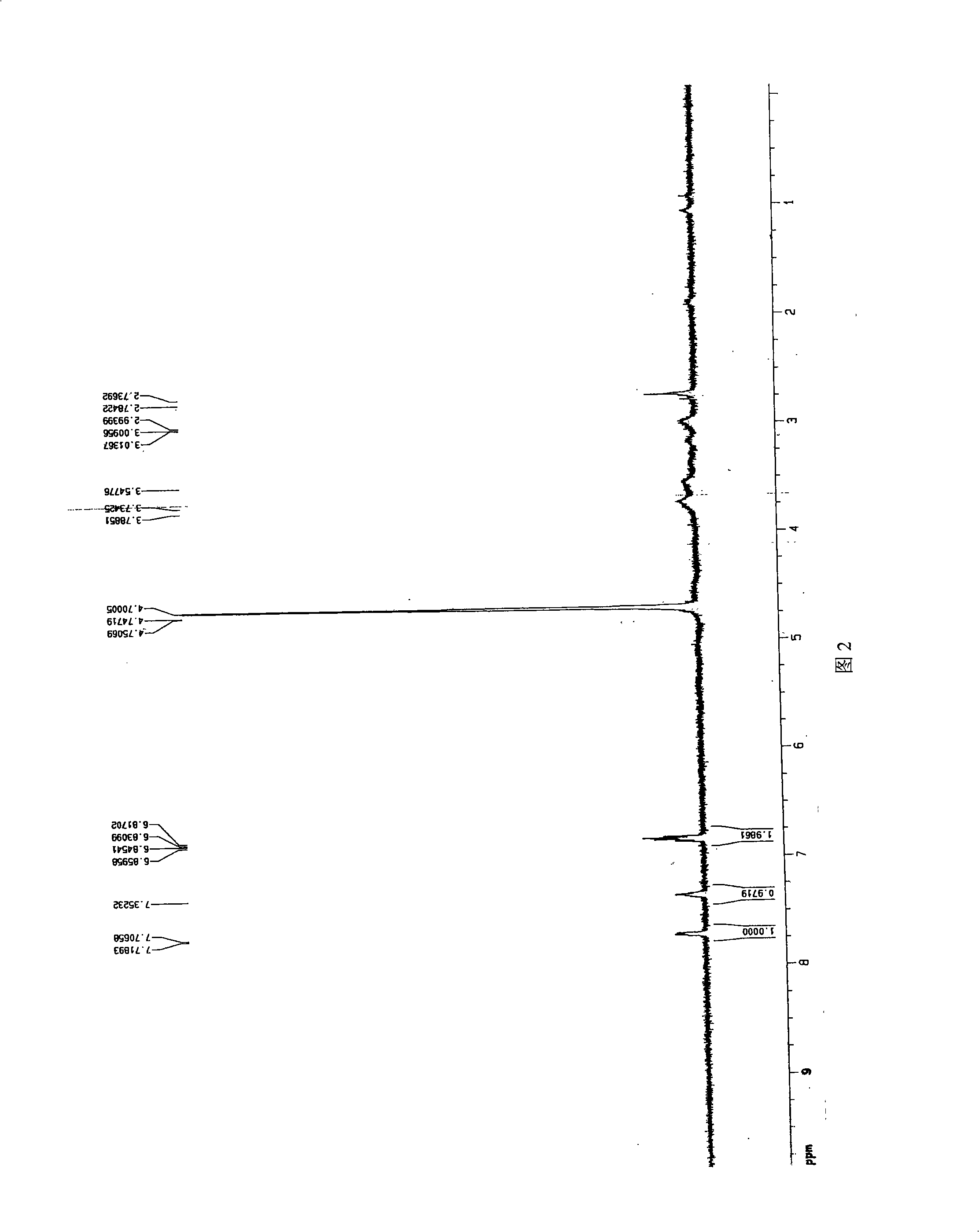
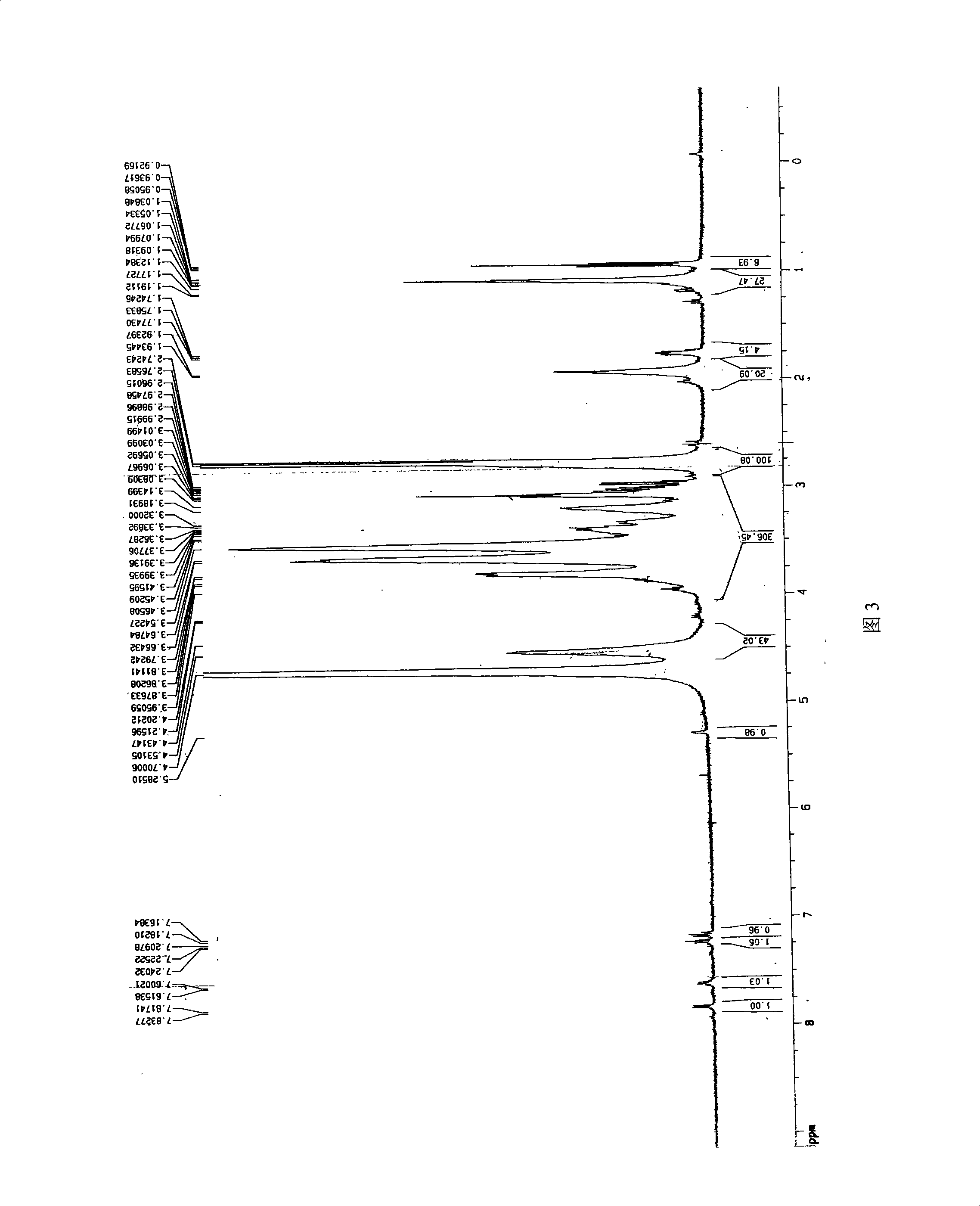
Image
Examples
Embodiment 1
[0026] (1) Preparation of chitosan oligosaccharide
[0027] Take commercially available chitosan (70% degree of deacetylation) with a molecular weight of 550kDa, stir for 2 hours at 55°C and pH5. : 100 (w / w) cellulase (produced by Shanghai Boao Biotechnology Co., Ltd.) was added to degrade chitosan. The degree of degradation of chitosan was controlled by viscosity method. The resulting chitosan degradation solution is filtered to remove impurities, and ultrafiltration is used for fractionation using ultrafiltration membranes with molecular weights of 10 kDa and 30 kDa. The ultrafiltrate with a molecular weight between 10kDa and 30kDa was freeze-dried to obtain chitosan oligosaccharide with a deacetylation degree of 70% and a molecular weight of 18.0kDa.
[0028] (2) Preparation of salicylic acid-g-chitooligosaccharide graft
[0029] Accurately weigh 0.2756g carbodiimide (EDC) and 0.0397g salicylic acid (Salicycleacid, SA) and place in 3ml absolute ethanol (A liquid), then a...
Embodiment 2-5
[0031] Salicylic acid-g-chitooligosaccharide grafts were prepared according to the synthetic recipe listed in Table 1, and other conditions were the same as in Example 1.
[0032] Table 1: Synthetic formulations of salicylic acid-chitooligosaccharide (SA-g-CSO) with different molecular weights and grafting ratios
[0033] Example
[0034] molecular weight
Embodiment 6
[0035] Embodiment 6: Determination of physicochemical properties of salicylic acid-g-chitooligosaccharide graft
[0036] The salicylic acid-g-chitooligosaccharide grafts prepared in Examples 1-5 were used as test objects.
[0037] A. The amino substitution degree of the obtained salicylic acid-g-chitooligosaccharide graft was determined by trinitrobenzenesulfonic acid method.
[0038]Take certain molecular weight chitosan oligosaccharides (0.5-9mg) of different weights, accurately weighed, dissolve in 2ml of distilled water respectively, add 2ml of 4% (w / v) sodium bicarbonate solution and 0.1% (w / v) of Trinitrobenzenesulfonic acid 2ml, incubated at 37°C for 2h. Add 2ml of 2N hydrochloric acid, shake well, drive away the air bubbles by ultrasonic, measure the absorbance value (A) at 344nm wavelength, and obtain the amino substitution standard curve of the molecular weight chitosan oligosaccharide. Weigh 4 mg of the salicylic acid graft of an appropriate amount of the molecula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com