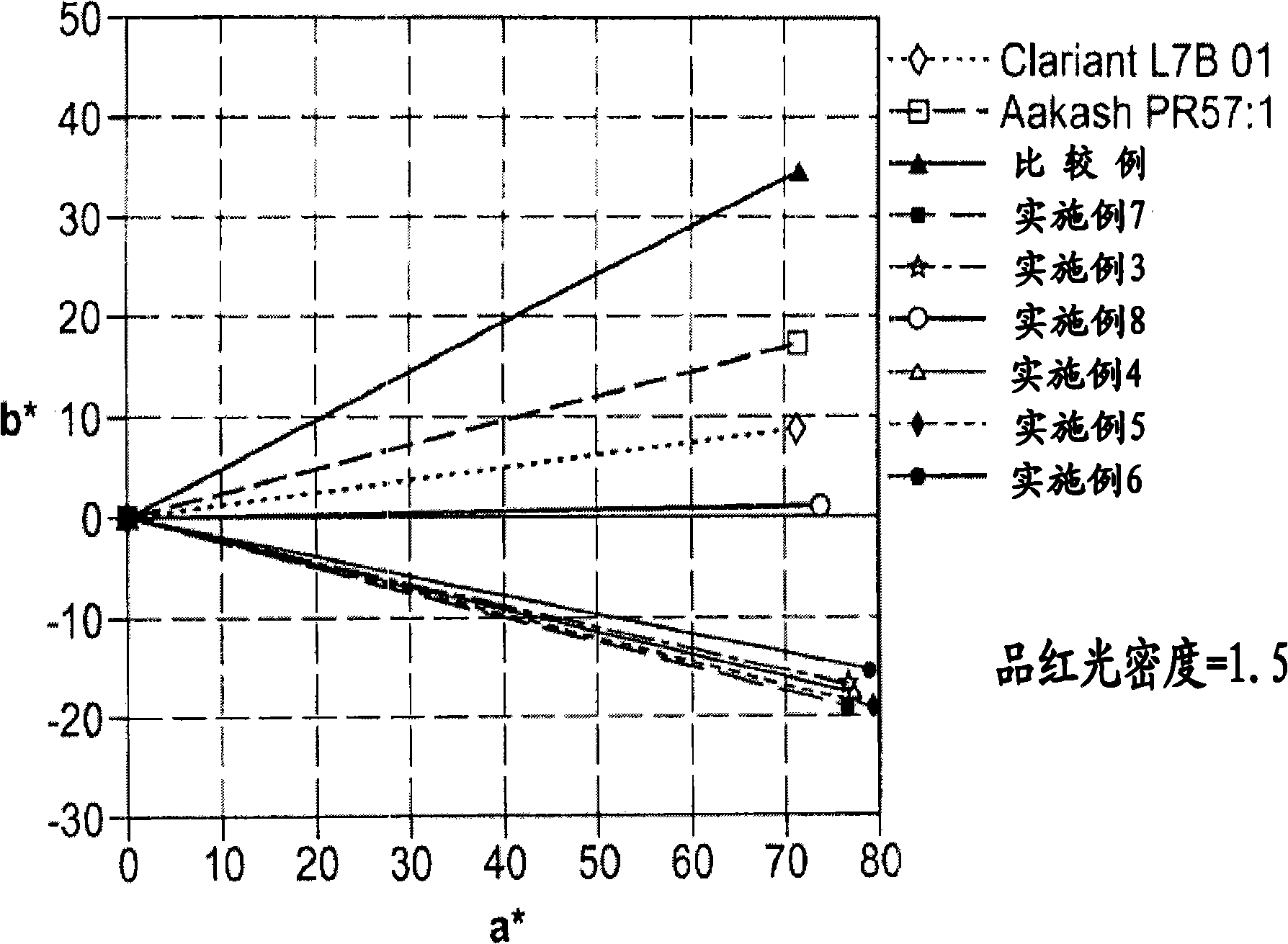
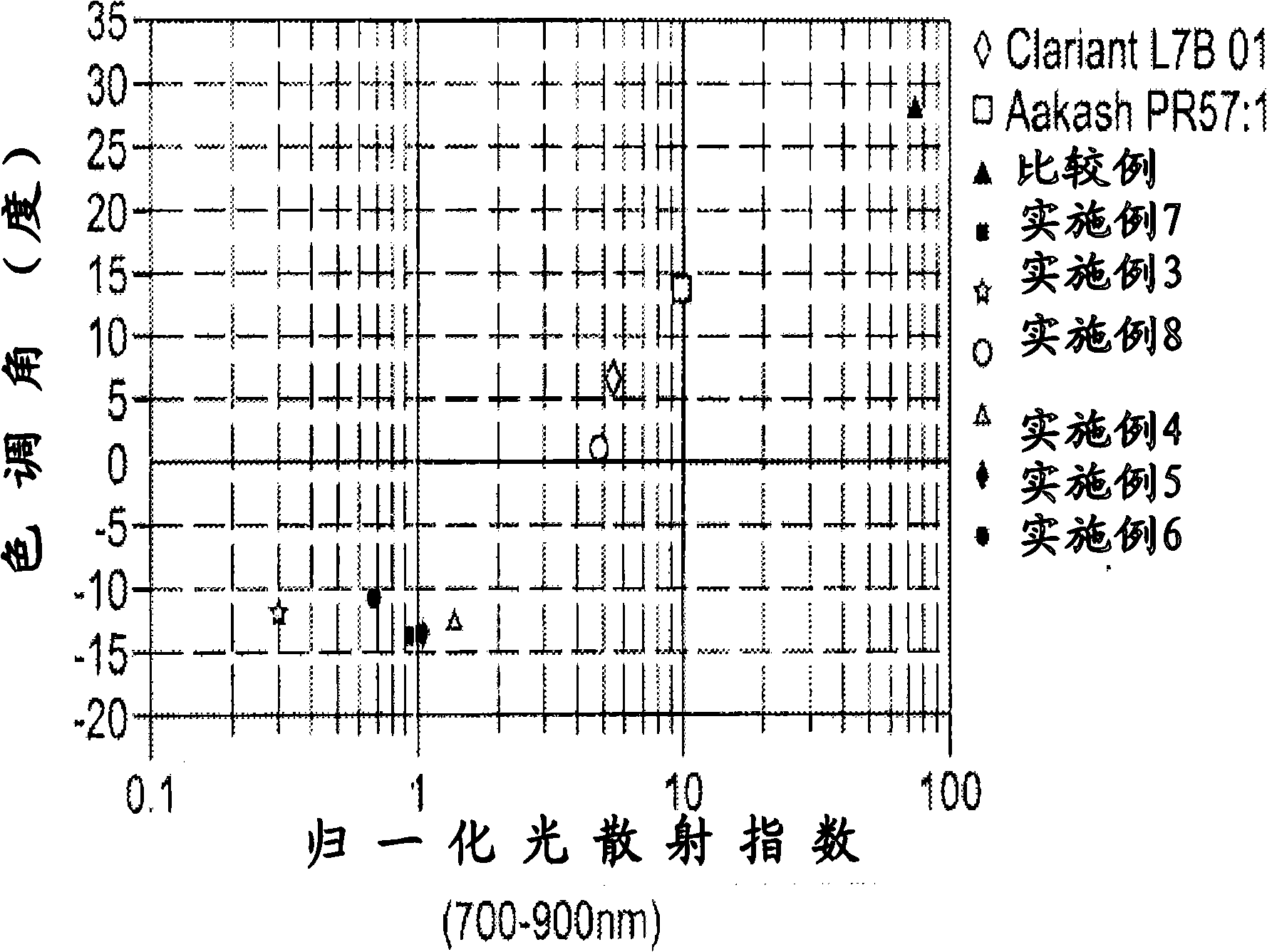
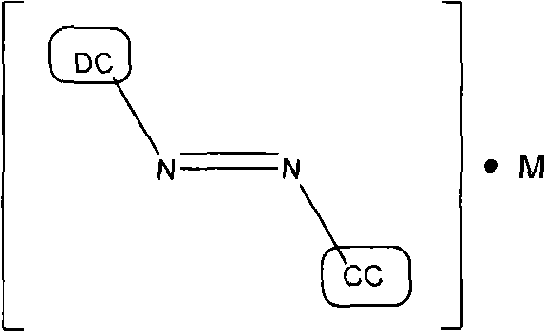
Nanosized particles of monoazo laked pigment
A technology of lake pigments and pigment particles, which is applied in the field of nano-scale pigment particle compositions, and can solve problems such as reducing the firmness of inks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The preparation of nanoscale particles of monoazo lake pigments, such as those listed in Table 7, involves at least one or more reaction steps. The diazotization reaction is a key step in the synthesis of monoazo lake pigments. First, a suitable aniline precursor (or diazo component DC, such as those listed in Table 1) is directly or indirectly converted into a diazonium salt, so The standard procedure includes, for example, the procedure of treating with a diazotization reagent, such as nitrous acid HNO 2 (eg, generated in situ by mixing sodium nitrite and dilute hydrochloric acid solutions) or nitrosylsulfuric acid (NSA, commercially available or prepared by mixing sodium nitrite and concentrated sulfuric acid). The acidic mixture of the diazonium salt obtained can be a solution or a suspension, preferably refrigerated in the embodiment, and metal salts (M n+ ), which will define the specific composition of the desired monoazo lake pigment product (as listed in Table...
Embodiment 1
[0148] Embodiment 1: Synthesis of Lithor rubine-potassium salt dye (precursor for preparing pigment red 57:1)
[0149] Diazotization procedure: In a 500 mL round bottom flask equipped with mechanical stirrer, thermometer and addition funnel, 2-Amino-5-methylbenzenesulfonic acid (8.82 g) was dissolved in 0.5M aqueous KOH (97.0 mL) . The resulting brown solution was cooled to 0 °C. Under the condition of keeping the temperature lower than 3°C, slowly add 20wt% sodium nitrite aqueous solution (3.28g NaNO 2 dissolved in 25mL of water). Concentrated HCl (10M, 14.15 mL) was added dropwise to the reddish-brown homogeneous mixture over 1 h keeping the internal temperature below 2°C. The mixture formed a light brown suspension, which was stirred for an additional 30 min after the addition of concentrated HCl was complete.
[0150] Coupling procedure: In a separate 2 L resin kettle, 3-hydroxy-2-naphthoic acid (8.86 g) was dissolved in a solution of KOH (8.72 g) in water (100 mL). A...
Embodiment 2
[0151] Embodiment 2: Synthesis of Lithor rubine-potassium salt dye (precursor for preparing pigment red 57:1)
[0152] Diazotization procedure: In a 500 mL round bottom flask equipped with a mechanical stirrer, thermometer and addition funnel, 2-amino-5-methylbenzenesulfonic acid (12.15 g) was dissolved in 0.5M aqueous KOH (135 mL). The resulting brown solution was cooled to 0 °C. Under the condition of keeping the temperature lower than -2°C, slowly add 20wt% sodium nitrite aqueous solution (4.52g NaNO 2 dissolved in 30mL water). Concentrated HCl (10M, 19.5 mL) was then slowly added dropwise over 1 hour while keeping the internal temperature below OC. The mixture formed a light brown suspension, which was stirred for an additional 30 min after the addition of concentrated HCl was complete.
[0153] Coupling procedure: In a separate 2 L resin kettle, 3-hydroxy-2-naphthoic acid (12.2 g) was dissolved in a solution of KOH (12.0 g) in water (130 mL). An additional 370 mL of w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com