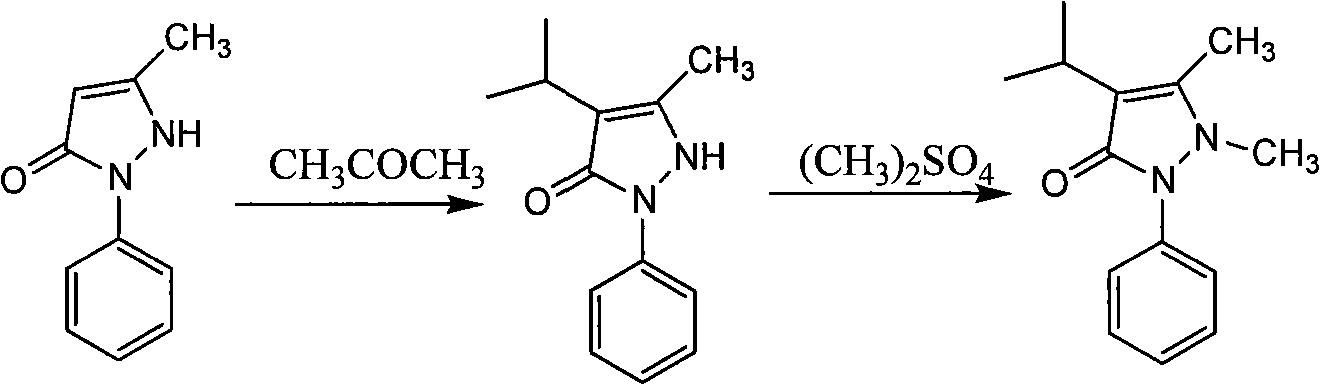
Low-voltage hydrogenation process of isopropyl pyrazolone
A technology of propyl pyrazolone and pyrazolone, which is applied in the field of chemical synthesis preparation technology, can solve problems such as safety and cost, and achieve the effects of fast reaction speed, cost reduction, and reduction of equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Put 30g of pyrazolone, 100g of acetone, and 1.5g of Raney nickel into the hydrogenation reaction kettle together. After four times of replacement with nitrogen, four times of replacement with hydrogen, the hydrogen is discharged to <0.1Mpa, and then the temperature is raised to At about 75°C, raise the pressure to 0.3Mpa to start the reaction, keep the pressure in the kettle at 0.3Mpa, and the reaction temperature at 80°C. After the reaction was completed, the reaction solution was lowered to 60°C, and the reaction solution was filtered out with a filter press, the temperature of the reaction solution was lowered, and it was crystallized at 0-5°C and then filtered to obtain 35 g of white powdery solid isopyrazolone. Yield 93.99%, content 99.21%.
Embodiment 2
[0028] Example 2: Put 50g of pyrazolone, 165g of acetone, and 2.5g of Raney's nickel into the hydrogenation reactor. After four times of nitrogen replacement, four times of hydrogen replacement, the hydrogen is discharged to <0.1Mpa, and then the temperature is raised to At about 75°C, raise the pressure to 0.32Mpa to start the reaction, keep the pressure in the kettle at 0.32Mpa, and the reaction temperature at 82°C. After the reaction was completed, the reaction solution was lowered to 60°C, and the reaction solution was filtered out with a filter press, the temperature of the reaction solution was lowered, and the crystallization was carried out at 0-5°C, followed by filtration to obtain 59 g of white powdery solid isopyrazolone. Yield 95.06%, content 99.03%.
Embodiment 3
[0029] Example 3: 50g of pyrazolone, 140g of acetone, and 2g of Raney nickel were put into the hydrogenation reaction kettle together, and after four times of replacement with nitrogen, four times of replacement with hydrogen, the hydrogen was discharged to <0.1Mpa, and then the temperature was raised to 75 ℃, raise the pressure to 0.3Mpa, start the reaction, keep the pressure in the kettle at 0.3Mpa, and the reaction temperature at 85℃. After the reaction was completed, the reaction liquid was lowered to 60°C, and the reaction liquid was filtered out with a filter press, the temperature of the reaction liquid was lowered, and the temperature of the reaction liquid was crystallized at 0-5°C, and then filtered to obtain 45 g of white powdery solid isopyrazolone. Yield 90.63%, content 99.48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com