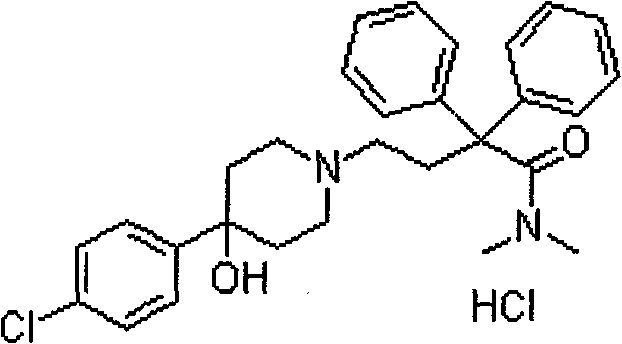
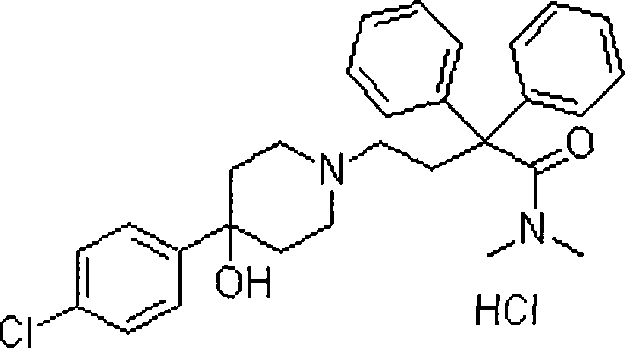
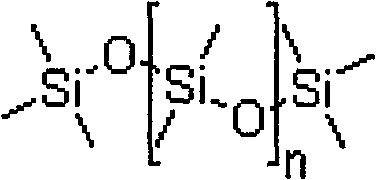Loperamide hydrochloride dimethicone compound rapidly disintegrating oral membrane
A technology of loperamide hydrochloride simethicone and loperamide hydrochloride, which can be applied in pharmaceutical formulas, active ingredients of artificially synthesized polymeric materials, and digestive system, and can solve slow disintegration, inconvenient carrying, and oral disintegration Problems such as complex preparation process of tablets and oral instant tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Determination of the dissolution time limit of loperamide hydrochloride simethicone compound oral instant film:
[0046] Take 10 sheets of medicine film randomly, one piece at a time, clamp one side of the medicine film with a clip with a jaw of about 2cm, and immerse the clip together in a water bath at 37°C, and use a stopwatch to time the medicine film from being immersed in water to dissolving And the time of breaking away from the clip is the melting time limit.
Embodiment 2
[0048] Loperamide Hydrochloride 1g
[0049] Simethicone 62.5g
[0050]Pullulan 50g
[0051] Maltodextrin 10g
[0052] Stevioside 2g
[0053] Polyethylene glycol-200 1g
[0054] Purified water 100g
[0055] Dissolve the above-mentioned amount of maltodextrin and stevioside in water to obtain an aqueous solution, then add the above-mentioned amount of amylopectin into the maltodextrin aqueous solution under stirring, fully stir and disperse to obtain a pullulan gel, and then add Polyethylene glycol-200 and stir evenly, finally add loperamide hydrochloride and simethicone and stir evenly to obtain loperamide hydrochloride simethicone gel, degas, uniformly add loperamide hydrochloride simethicone The gel is coated on a flat plate, heated and dried at 75-80° C., and cut to obtain the loperamide hydrochloride simethicone compound oral instant film. The film is white, flexible, melts and disperses quickly at the entrance, has a good taste and sweet taste, and the melting time i...
Embodiment 3
[0057] Loperamide Hydrochloride 1g
[0058] Simethicone Compound 62.5g
[0059] Hydroxypropyl methylcellulose (3 centipoise) 80g
[0060] Maltodextrin 40g
[0061] Sucralose 3g
[0062] Lemon essence 2g
[0063] Glycerin 1g
[0064] Ethylparaben 1g
[0065] Citric acid 2g
[0066] lemon yellow 2g
[0067] Purified water 150g
[0068] First dissolve the above-mentioned amount of maltodextrin, citric acid and sucralose in water to obtain an aqueous solution, then add the above-mentioned amount of hydroxypropyl methylcellulose (3 centipoise) into the aqueous solution under stirring, and stir thoroughly Disperse to obtain hydroxypropyl methylcellulose gel, take the above-mentioned amount of ethylparaben, heat and dissolve it with glycerin, then add it to the hydroxypropyl methylcellulose gel and stir evenly, and finally add loperamide hydrochloride , simethicone, lemon essence and tartrazine and stir evenly to obtain loperamide hydrochloride simethicone gel, degas, and ev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com