Hydroxyl group functionalization carbon nanotube for polyurethane chain-expanding agent and method for preparing same
A technology of carbon nanotubes and polyurethane, applied in the field of materials, can solve the problems of destroying carbon nanotubes and affecting the performance of carbon nanotubes, and achieve the effects of improving dispersion, increasing interfacial bonding force, and improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
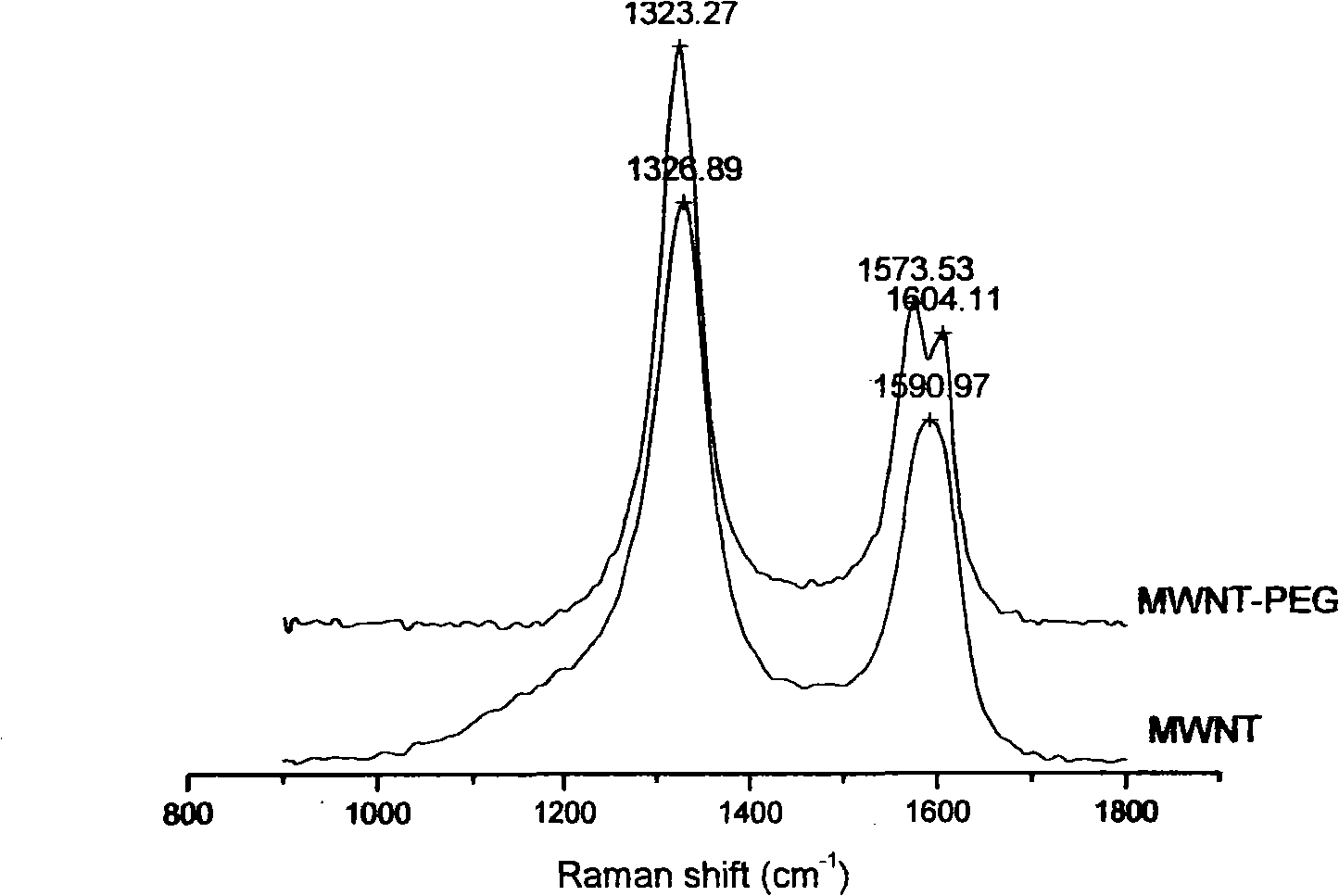
[0028] Example 1: Polyethylene glycol (molecular weight 2000) PEG-2000 modified multi-walled carbon nanotubes to prepare hydroxyl functionalized multi-walled carbon nanotubes
[0029] (a) Add 40ml of 98% nitric acid, 40ml of 96% sulfuric acid and 120ml of dichloromethane into the three-necked flask successively, stir and cool to 0-5°C. 30 ml of dichloromethane dissolved in 50 g of polyethylene glycol 2000 was slowly added dropwise. The temperature is maintained at 5-8°C. After the dropwise addition, the reaction was continued for 1 h. After the reaction, the reaction mixture was poured into ice water, the dichloromethane layer was separated, and washed continuously with water and 50 wt% aqueous sodium bicarbonate until neutral. After removing the solvent, polyethylene glycol 2000 with one end terminated by a nitrate group was obtained.
[0030] (b) Put 10mmol of polyethylene glycol 2000 capped with nitrate group, 21mmol of sodium azide, and 15ml of distilled water in a three...
Embodiment 2
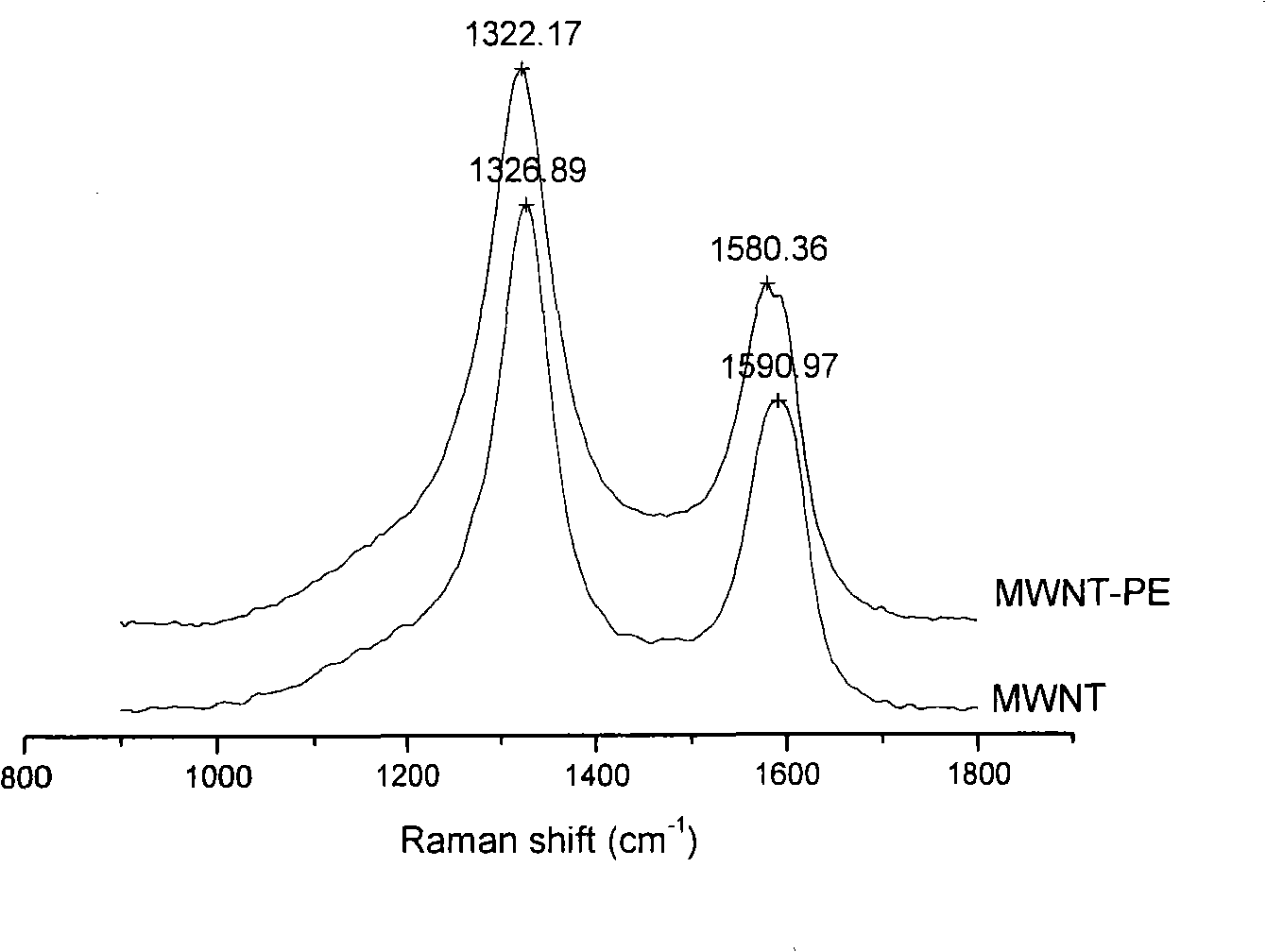
[0032] Example 2: Preparation of hydroxyl-functionalized single-walled carbon nanotubes by modifying single-walled carbon nanotubes with polytetrahydrofuran diol (molecular weight: 2000)
[0033](a) Add 40ml of 98% nitric acid, 40ml of 96% sulfuric acid and 120ml of dichloromethane into the three-necked flask successively, stir and cool to 0-5°C. 30 ml of dichloromethane dissolved in 50 g of polytetrahydrofuran diol 2000 was slowly added dropwise. The temperature is maintained at 5-8°C. After the dropwise addition, the reaction was continued for 1 h. After the reaction, the reaction mixture was poured into ice water, the dichloromethane layer was separated, and washed continuously with water and 50 wt% aqueous sodium bicarbonate until neutral. After removing the solvent, polytetrahydrofuran diol whose end is terminated by a nitrate group is obtained.
[0034] (b) Put 10mmol of polytetrahydrofuran diol terminated by nitrate ester groups, 21mmol of sodium azide, and 15ml of di...
Embodiment 3
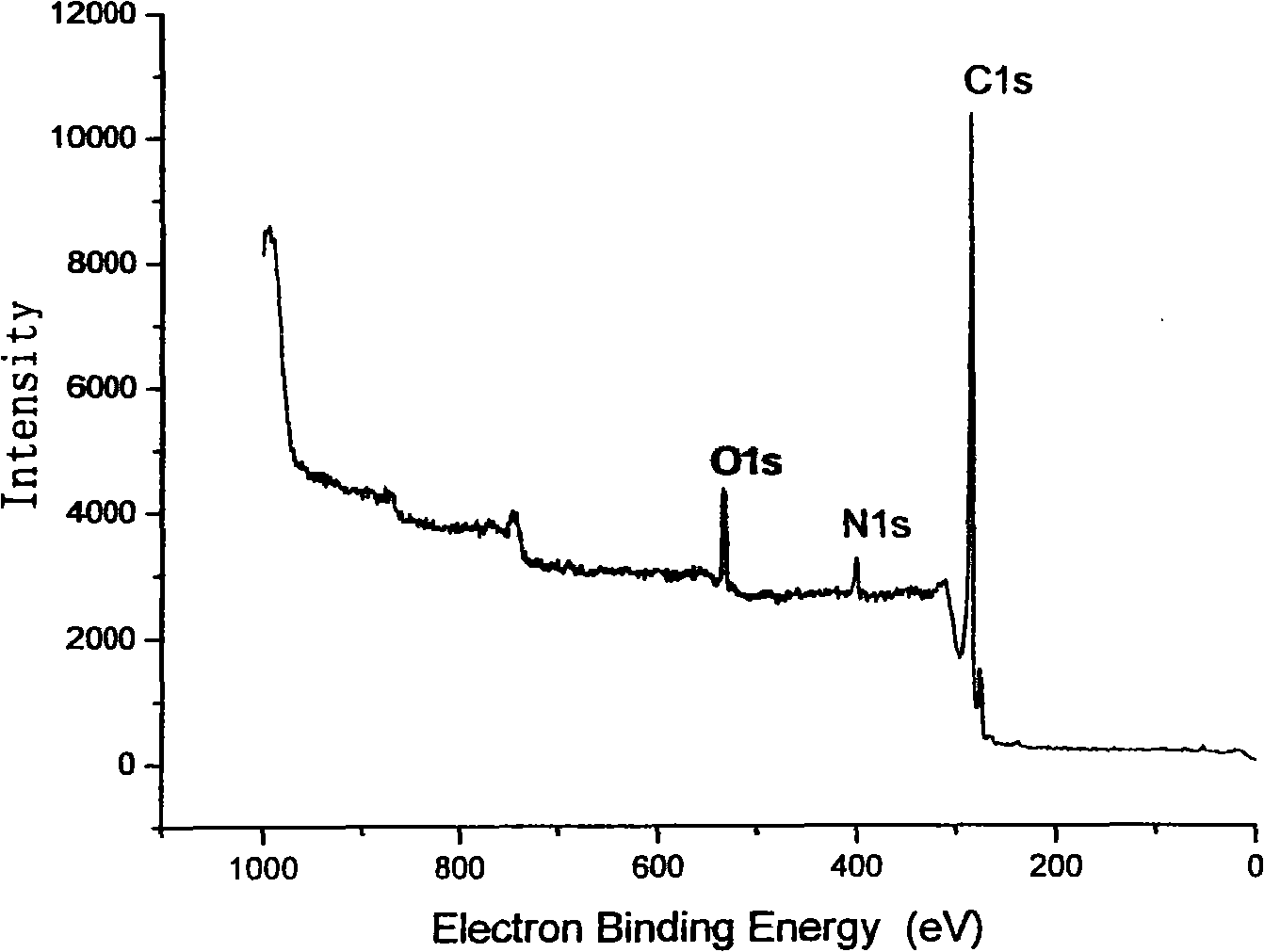
[0036] Example 3: Pentaerythritol modified multi-walled carbon nanotubes to prepare hydroxyl-functionalized multi-walled carbon nanotubes
[0037] (a) Add 40ml of 98% nitric acid, 40ml of 96% sulfuric acid and 120ml of dichloromethane into the three-necked flask successively, stir and cool to 0-5°C. 30ml of dichloromethane dissolved in 4g of pentaerythritol was slowly added dropwise. The temperature is maintained at 5-8°C. After the dropwise addition, the reaction was continued for 1 h. After the reaction, the reaction mixture was poured into ice water, the dichloromethane layer was separated, and washed continuously with water and 50 wt% aqueous sodium bicarbonate until neutral. After removal of the solvent, pentaerythritol with one end capped by a nitrate group is obtained.
[0038] (b) Put 10mmol of pentaerythritol capped with a nitrate group at one end, 21mmol of sodium azide, and 15ml of distilled water in a three-necked flask, heat to 90°C while stirring, react for 24h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com