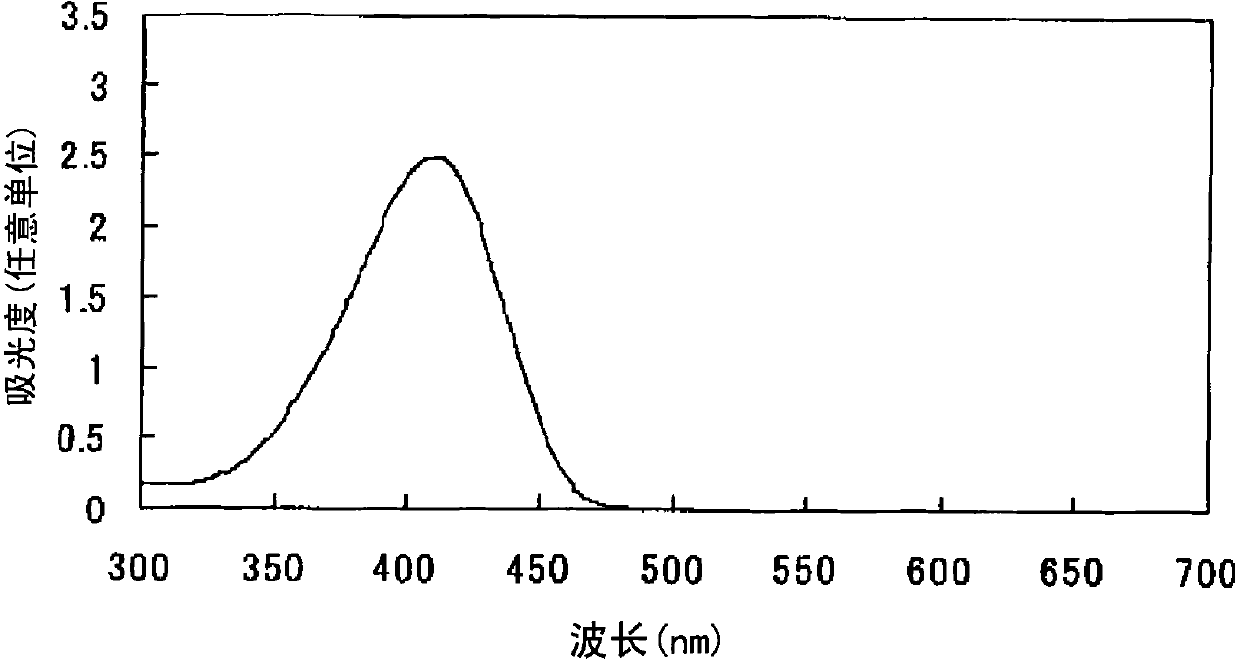
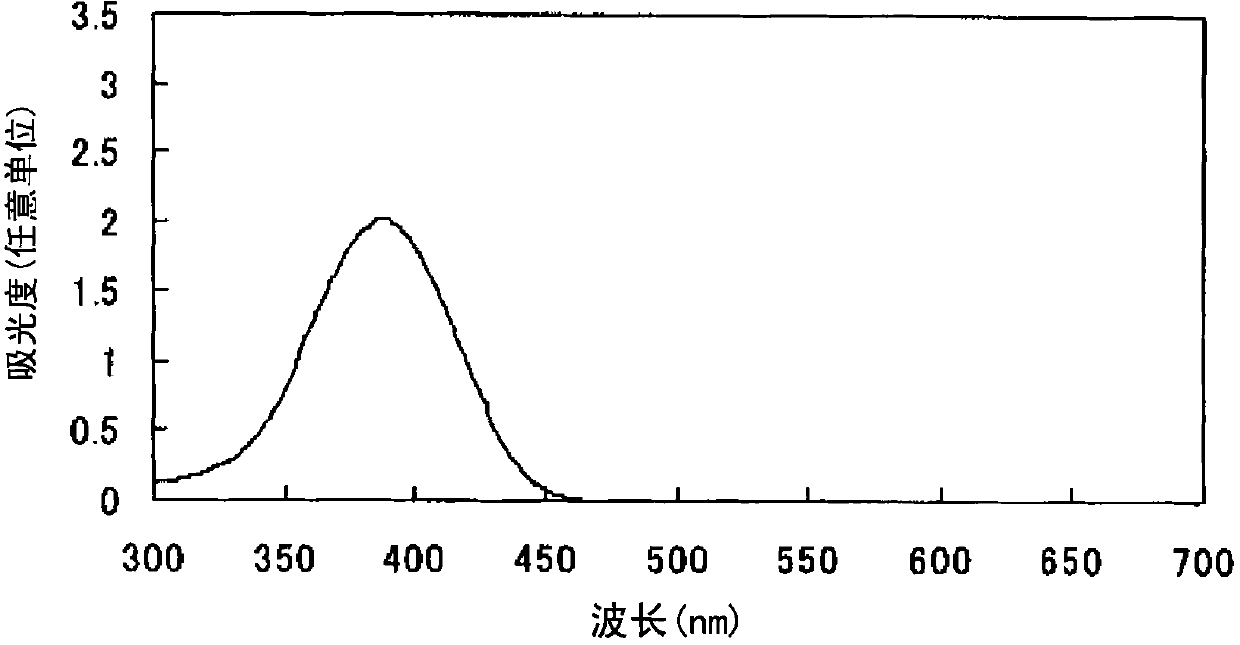
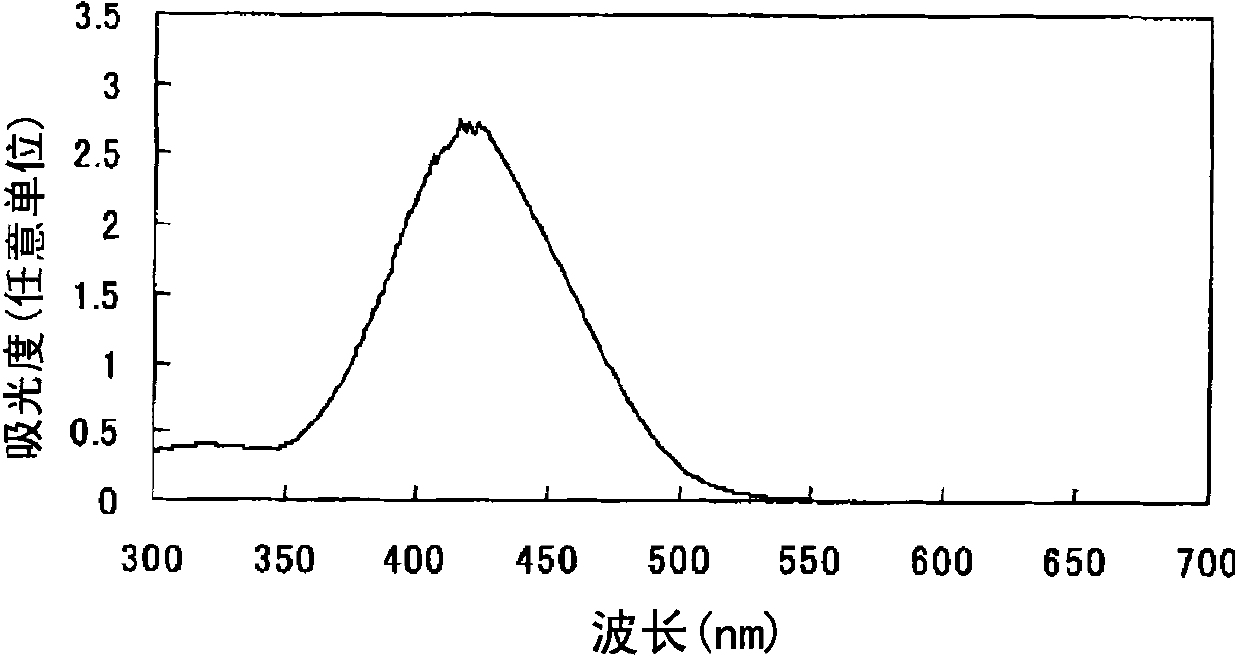
Azo compound or salts thereof
A technology of azo compound and carbon number, applied in the field of azo compound or its salt, to achieve the effect of good chroma and high color concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] After adding 300 parts of water to 30 parts of 2,2'-benzidine disulfonic acid (water content 30%) represented by formula (a-1), the pH was adjusted to 7-8 with 30% aqueous sodium hydroxide solution under ice cooling. The following operations were carried out under ice cooling. Add 12.6 parts of sodium nitrite and stir for 30 minutes. 38.1 parts of 35% hydrochloric acid were added a little at a time to form a brown solution and stirred for 2 hours. Dissolve 5.3 parts of sulfamic acid in 57.4 parts of water to form an aqueous solution, add the aqueous solution to the reaction solution, and stir to obtain a suspension containing diazonium salt.
[0074]
[0075] After adding 372 parts of water to 18.6 parts of N,N'-dimethylbarbituric acid represented by the formula (c-1), the pH was adjusted to 8-9 with a 30% aqueous sodium hydroxide solution under ice cooling.
[0076]
[0077] The following operations were performed under ice-cooling. The above-mentioned alkali ...
Embodiment 2
[0098] After adding 360 parts of water to 36 parts of 2,2'-benzidine disulfonic acid (water content 30%) represented by formula (a-1), the pH was adjusted to 7-8 with 30% aqueous sodium hydroxide solution under ice cooling. The following operations were carried out under ice cooling. Add 25.3 parts of sodium nitrite and stir for 30 minutes. 68.6 parts of 35% hydrochloric acid were added a little at a time to form a brown solution and stirred for 2 hours. Dissolve 20.7 parts of sulfamic acid in 206.6 parts of water to form an aqueous solution, add the aqueous solution to the reaction solution, and stir to obtain a suspension containing diazonium salt.
[0099] After adding 379.8 parts of water to 25.3 parts of thiobarbituric acid represented by the formula (c-2), the pH was adjusted to 8-9 with a 30% aqueous sodium hydroxide solution under ice cooling.
[0100]
[0101] The following operations were performed under ice-cooling. The above-mentioned alkaline aqueous solutio...
Embodiment 3
[0105] After adding 375 parts of water to 25 parts of 2,2'-benzidine disulfonic acid (water content 30%) represented by formula (a-1), the pH was adjusted to 7-8 with 30% aqueous sodium hydroxide solution under ice cooling. The following operations were carried out under ice cooling. Add 10.2 parts of sodium nitrite and stir for 30 minutes. 31.8 parts of 35% hydrochloric acid was added a little at a time to form a brown solution and stirred for 2 hours. Dissolve 4.8 parts of sulfamic acid in 47.8 parts of water to form an aqueous solution, add the aqueous solution to the reaction solution, and stir to obtain a suspension containing diazonium salt.
[0106] After adding 295 parts of water and 295 parts of acetone to 29.5 parts of N-(n-butyl)-N'-(4-methoxyphenyl) barbituric acid represented by formula (c-3), use 30 parts under ice cooling The pH was adjusted to 10 with aqueous sodium hydroxide solution.
[0107]
[0108] The following operations were performed under ice-co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com