Preparation method for adefovir dipivoxil ester waterless crystallization article, prepared adefovir dipivoxil ester waterless crystallization article and uses thereof
A technique for adefovir dipivoxil and anhydrous crystallization, which is applied in the field of preparation of adefovir dipivoxil anhydrous crystallization, and can solve problems such as general crystallization yield and crystallization purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
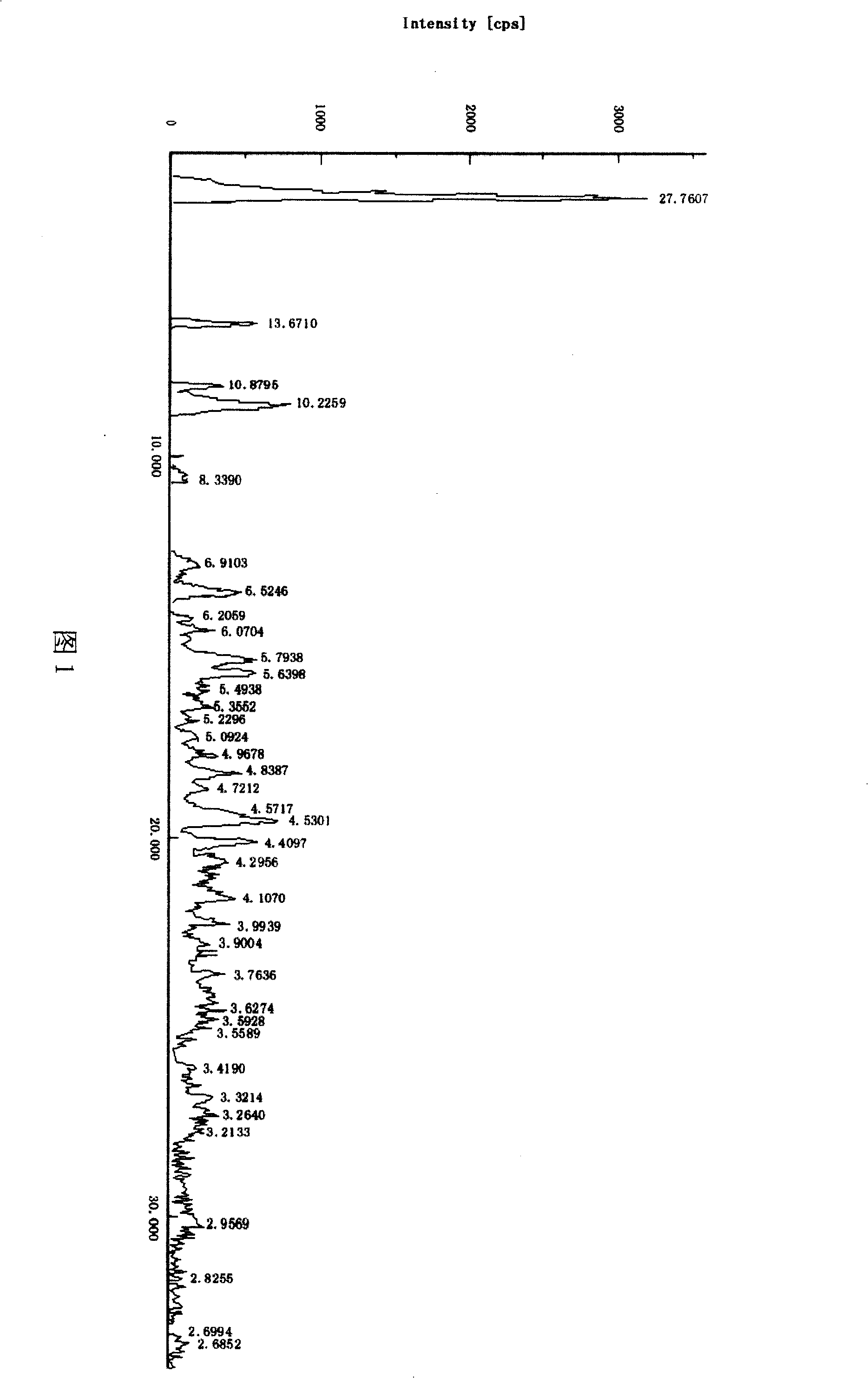
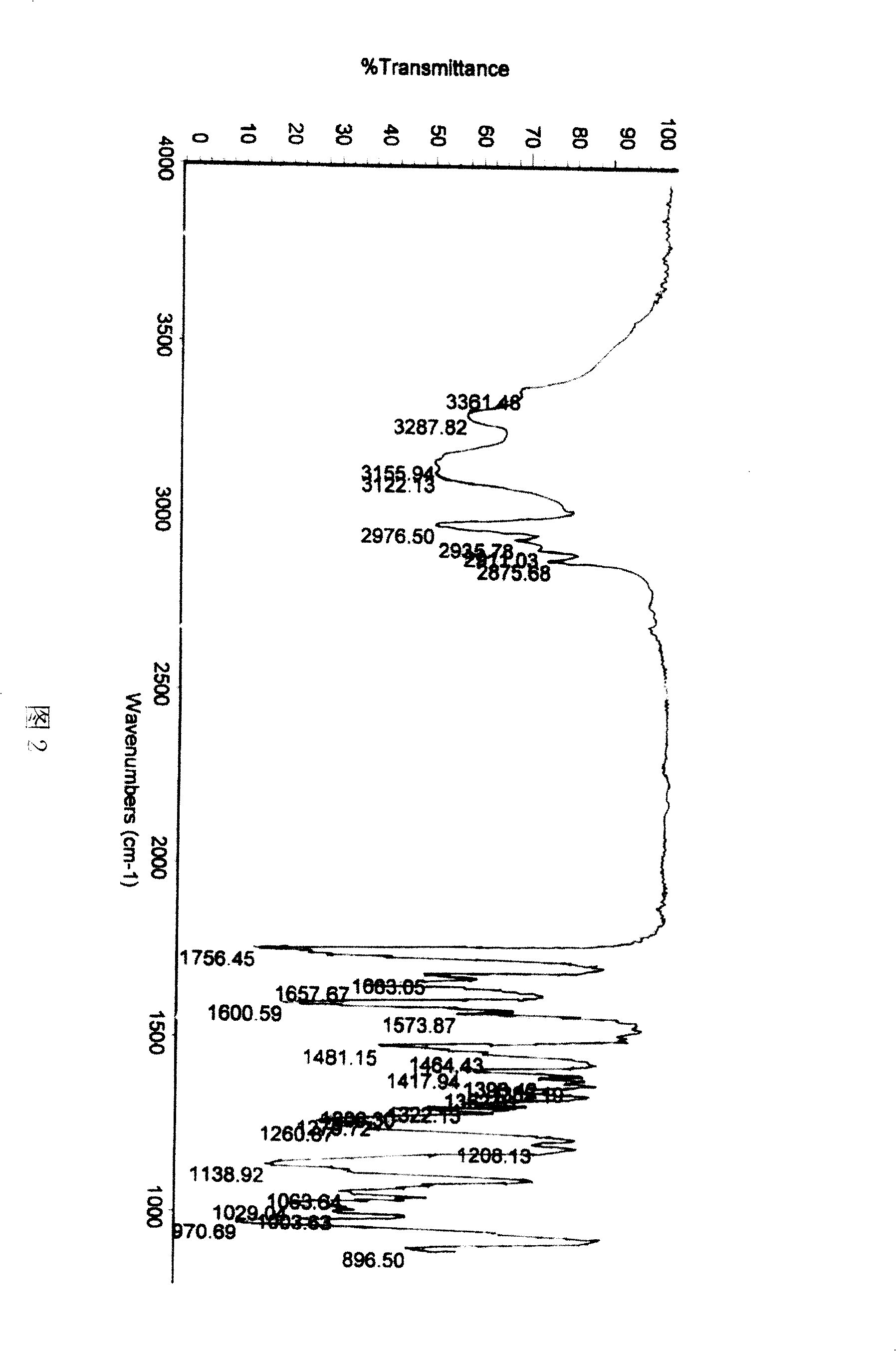
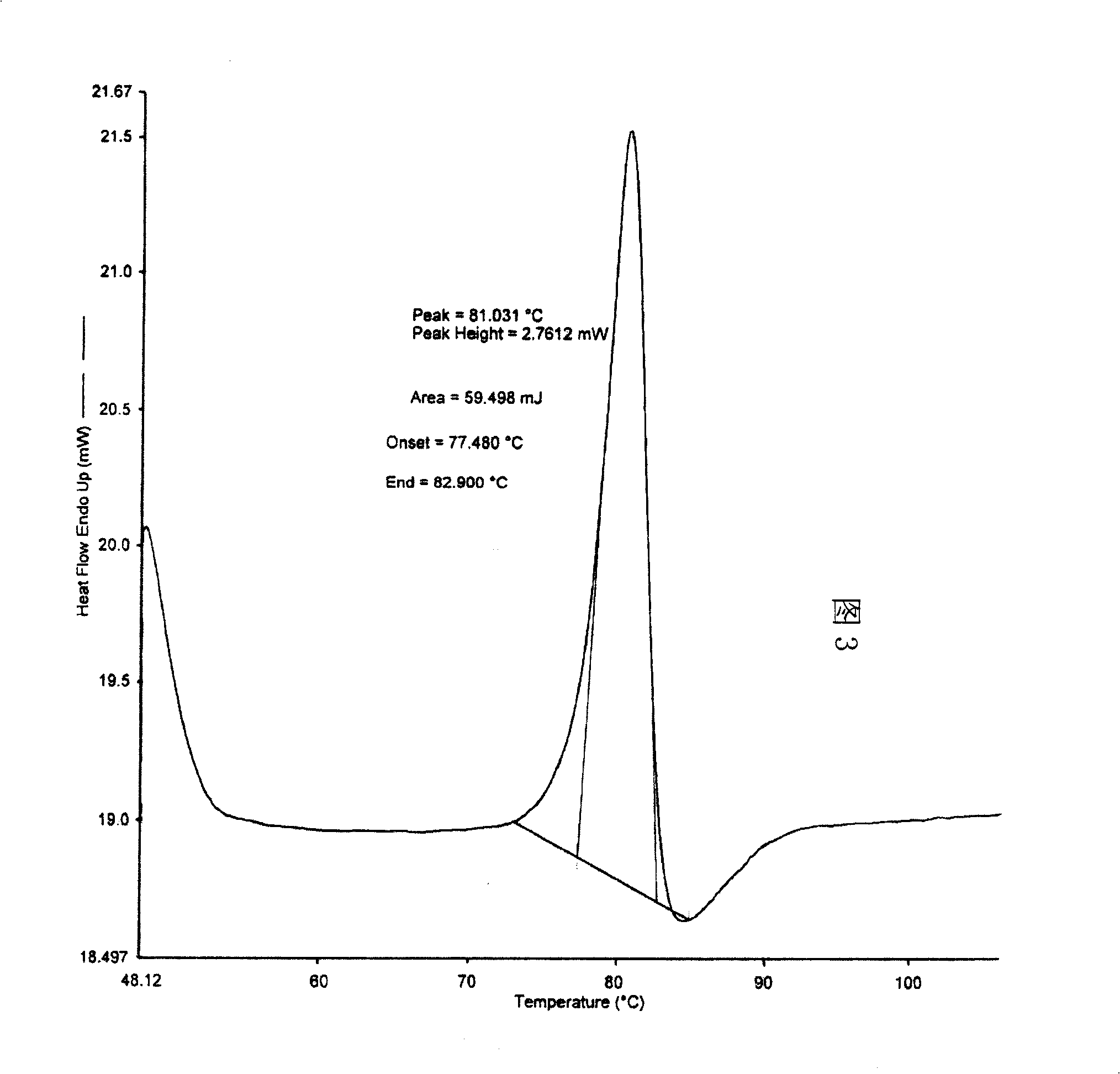
[0015] Dissolve 100g of crude adefovir dipivoxil in 200ml of 95% ethanol, heat to 40°C to dissolve, then cool to 10°C, and put it into a constant pressure funnel. Add 3000ml of isopropyl ether into a 5000ml three-necked flask, stir and cool to 2°C, and then add the adefovir dipivoxil solution to the isopropyl ether dropwise. Then it was kept at 2°C and stirred for 25 minutes, filtered, the filter cake was washed with isopropyl ether, and dried under reduced pressure at 50°C to obtain 96.5 g of adefovir dipivoxil as anhydrous crystals. The content of 99.8% is determined by HPLC. The X-ray powder diffraction spectrum is shown in Figure 1, the infrared absorption spectrum is shown in Figure 2, and the DSC diagram is shown in the attached file. image 3 .
Embodiment 2
[0017] Dissolve 100g of crude adefovir dipivoxil in 200ml of 95% ethanol, heat to 45°C to dissolve, then lower the temperature to 12°C, and put it into a constant pressure funnel. Add 3000ml of n-butyl ether into a 5000ml three-necked flask, stir and cool to 3°C, and then add the adefovir dipivoxil solution to the n-butyl ether dropwise. Then it was kept at 3°C and stirred for 30 minutes, filtered, and the filter cake was washed with n-butyl ether and dried under reduced pressure at 45°C to obtain 98 g of adefovir dipivoxil as anhydrous crystals. The content of 99.6% is determined by HPLC. The X-ray powder diffraction spectrum is shown in Figure 1, the infrared absorption spectrum is shown in Figure 2, and the DSC diagram is shown in the attached file. image 3 .
Embodiment 3
[0019] Dissolve 100g of crude adefovir dipivoxil in 200ml of 95% ethanol, heat to 50°C to dissolve, then lower the temperature to 11°C, and put it into a constant pressure funnel. Add 3000ml of sec-butyl ether into a 5000ml three-necked flask, stir and cool to 4°C, and then add the adefovir dipivoxil solution to the sec-butyl ether dropwise. Then it was kept at 2°C and stirred for 0.5hr, filtered, the filter cake was washed with sec-butyl ether, and dried under reduced pressure at 50°C to obtain 98.3g of adefovir dipivoxil as anhydrous crystals. The content of 99.8% is determined by HPLC. The X-ray powder diffraction spectrum is shown in Figure 1, the infrared absorption spectrum is shown in Figure 2, and the DSC diagram is shown in the attached file. image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com