Target-specificity dual-mutant amalgamation protein
A fusion protein, target-specific technology, applied in the field of target-specific double mutant fusion proteins, can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of mGnRH-PE38m4a fusion protein
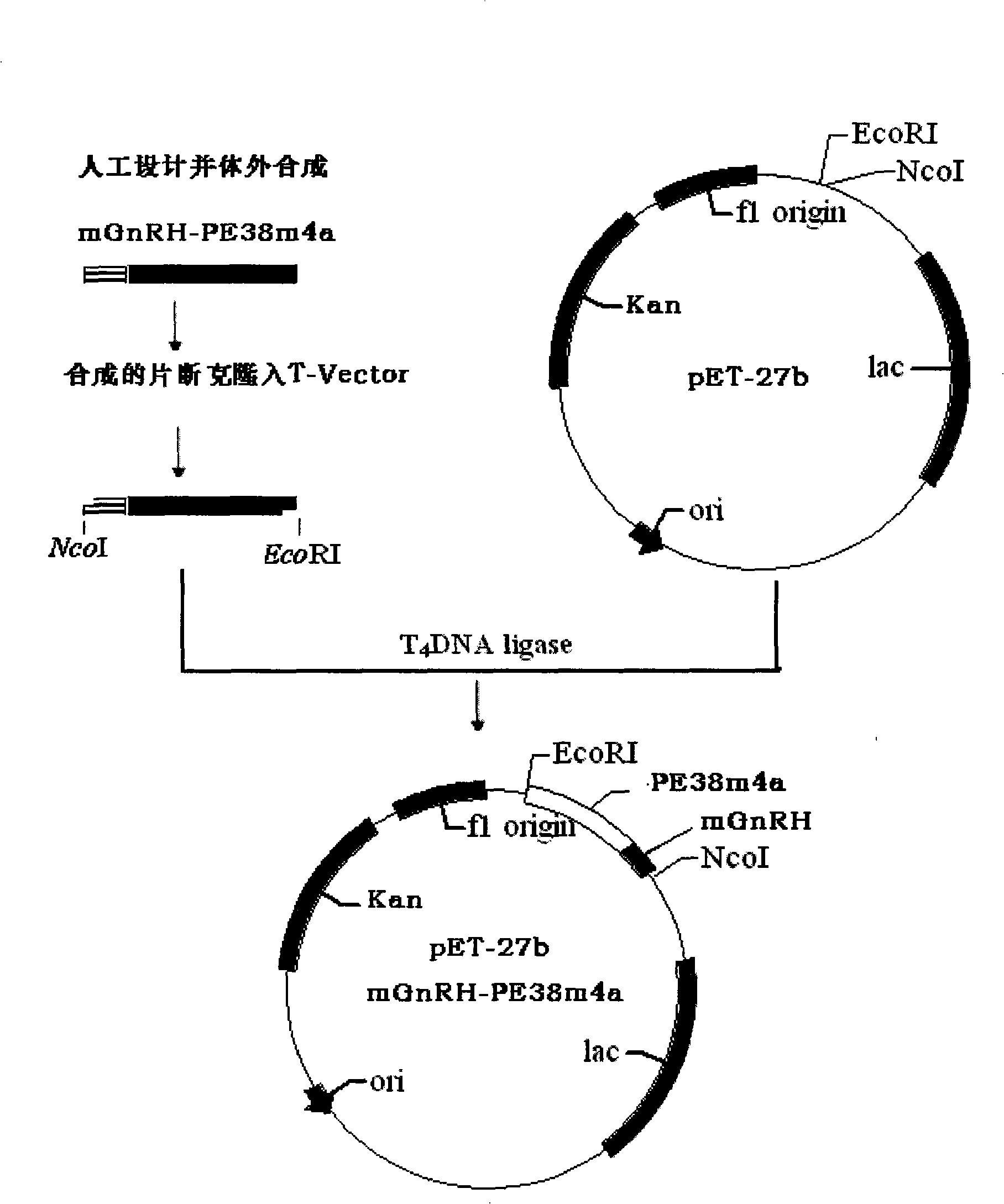
[0050] A. Construction and identification of recombinant expression plasmids
[0051] a. Preparation of mGnRH-PE38m4a double-stranded gene: referring to the existing literature, the double-stranded nucleotide sequence of mGnRH-PE38m4a shown in SEQ ID NO. ). in T 4 In the presence of ligase (Promega), it was directly cloned into the PGEM-T vector, and transformed into E. coli JM105 to obtain an engineered strain containing the PGEM-T / mGnRH-PE38m4a plasmid.
[0052] b. Preparation of mGnRH-PE38m4a expression plasmid: extract the PGEM-T / mGnRH-PE38m4a plasmid, and use NcoI and EcoRI endonuclease double digestion to collect the mGnRH-PE38m4a gene fragment, in T 4 In the presence of ligase (Promega), it was directly cloned into the pET27 vector digested with the same restriction enzyme, and transformed into E. coli JM105 to obtain an engineered strain containing the pET27-mGnRH-PE38m4a plasmid.
[0053] c. Screening...
Embodiment 2
[0058] Example 2: 125 Target specificity and biological activity analysis of I-labeled native GnRH and mGnRH-PE38m4a fusion proteins
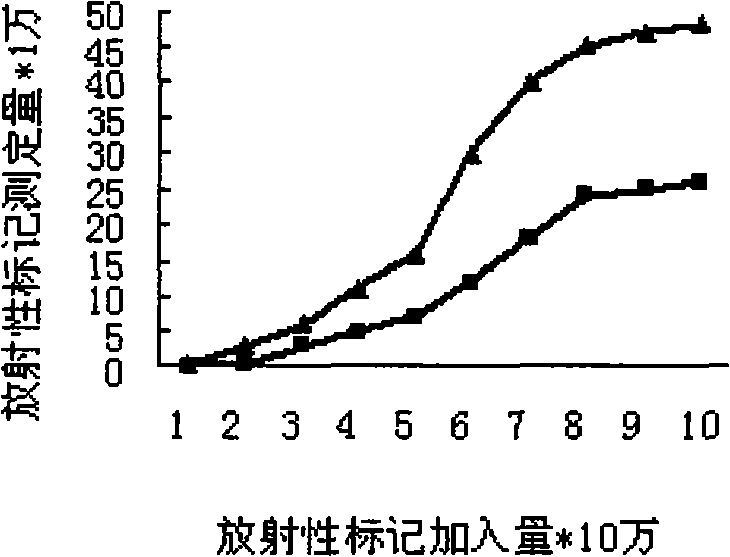
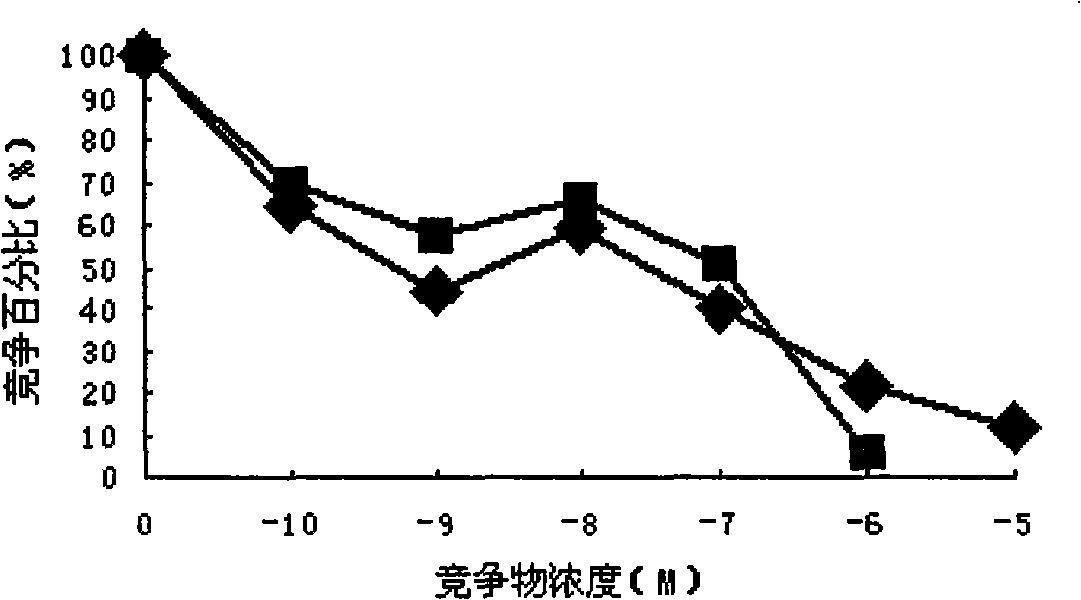
[0059] A. Binding ability test:
[0060] a. GnRH and mGnRH-PE38m4a 125 I labeling: Weigh 1 mg of Lodogen and dissolve it in 0.5 ml of chloroform, add 50 μl (100 μg) to the bottom of the test tube, dry it with nitrogen, add 0.4 ml of semi-finished polypeptide or protein without protective agent, add Na 125 1 5mCi was reacted at room temperature for 12min, and the reaction process was continuously shaken to make it fully react.
[0061] 125 The I-labeled mixture was separated and purified by Sepharyl S-200 HR gel column (1×50 cm), each tube was collected, and the tube with the highest radioactive intensity was selected for experiment.
[0062] b. Cancer cell and normal cell culture: RPMI-1640 complete medium in 5% CO 2 , 37 ℃ conditions of adherent monolayer culture and count. Add the same CPM to each cell well 125 I-labeled GnRH, mGnRH-PE...
Embodiment 3
[0069] Example 3: Cytotoxicity test:
[0070] The quantified samples were filtered and sterilized, and different amounts of samples were added to each cell well according to the equal dilution method to make the total volume 100 μl, 5% CO. 2 , incubate at 37°C for 12h, add 100μl MTT staining reagent to each well of the culture plate, 5% CO 2 , continue to culture at 37°C for 4h, measure the absorbance at 490nm wavelength, and calculate the concentration of recombinant toxin on 50% cell death of various tumor and normal cells (IC 50 ), while using natural Pseudomonas aeruginosa exotoxin A (PEA, Sigma) as a control to compare the activity changes of fusion toxin and natural PEA. The results are shown in Table 1.
[0071] Table 1: IC of purified mGnRH-PE38m4a and native PEA on tumor and normal cells 50
[0072]
[0073] Table 1 shows the cytotoxicity of purified mGnRH-PE38m4a and native PEA against certain tumor cells and certain normal cell cultures. IC 50 Values refe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com