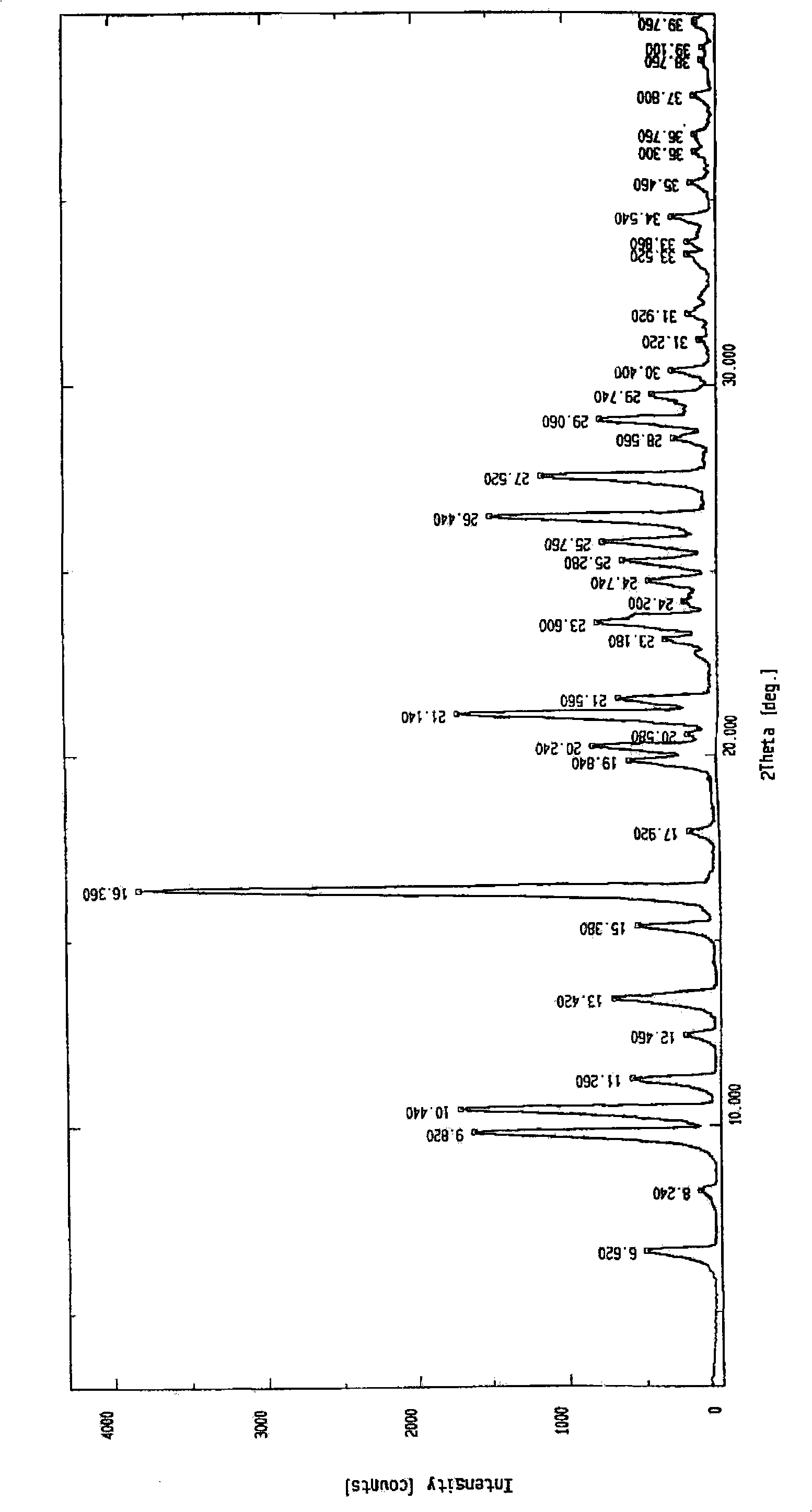
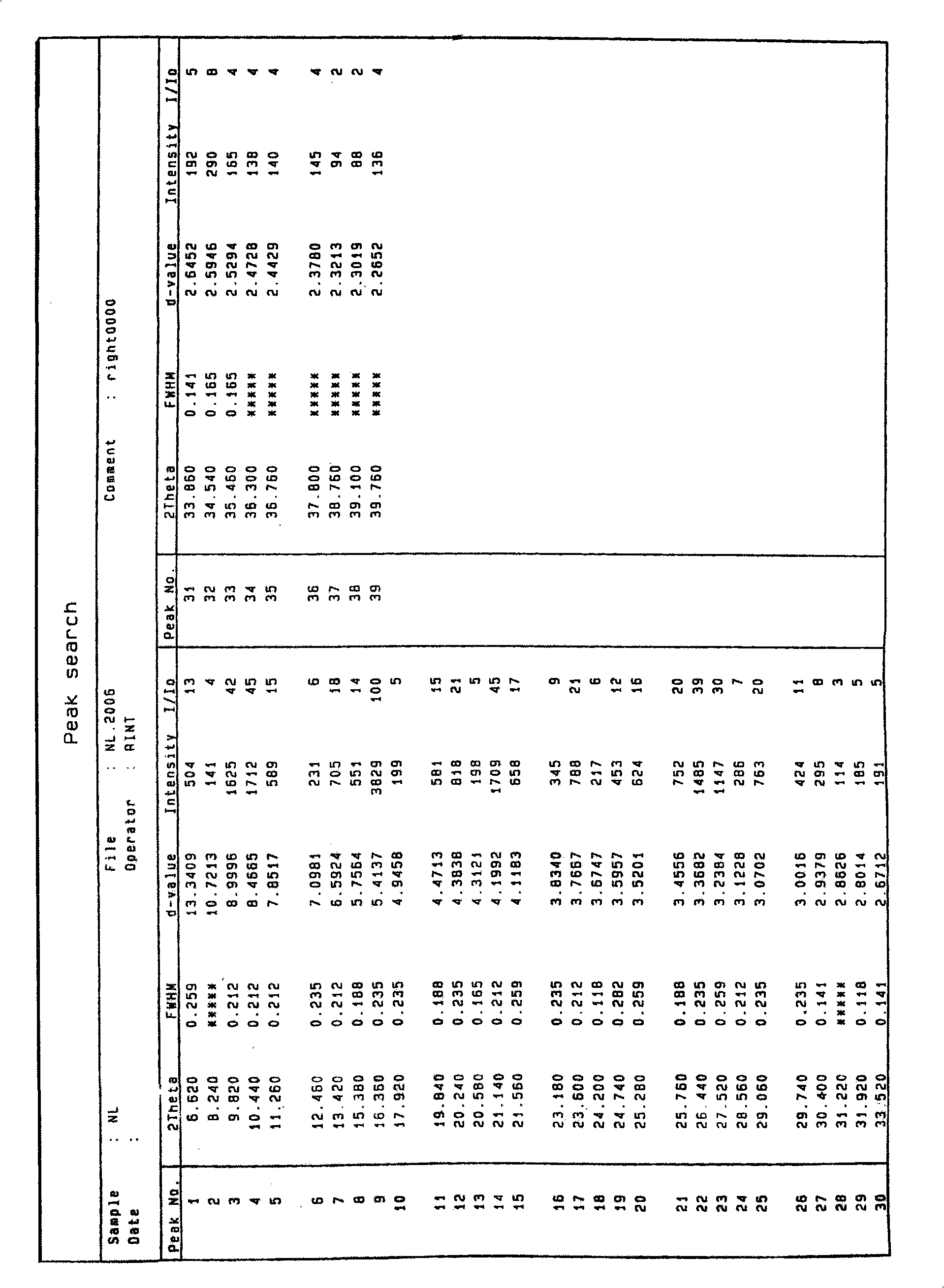
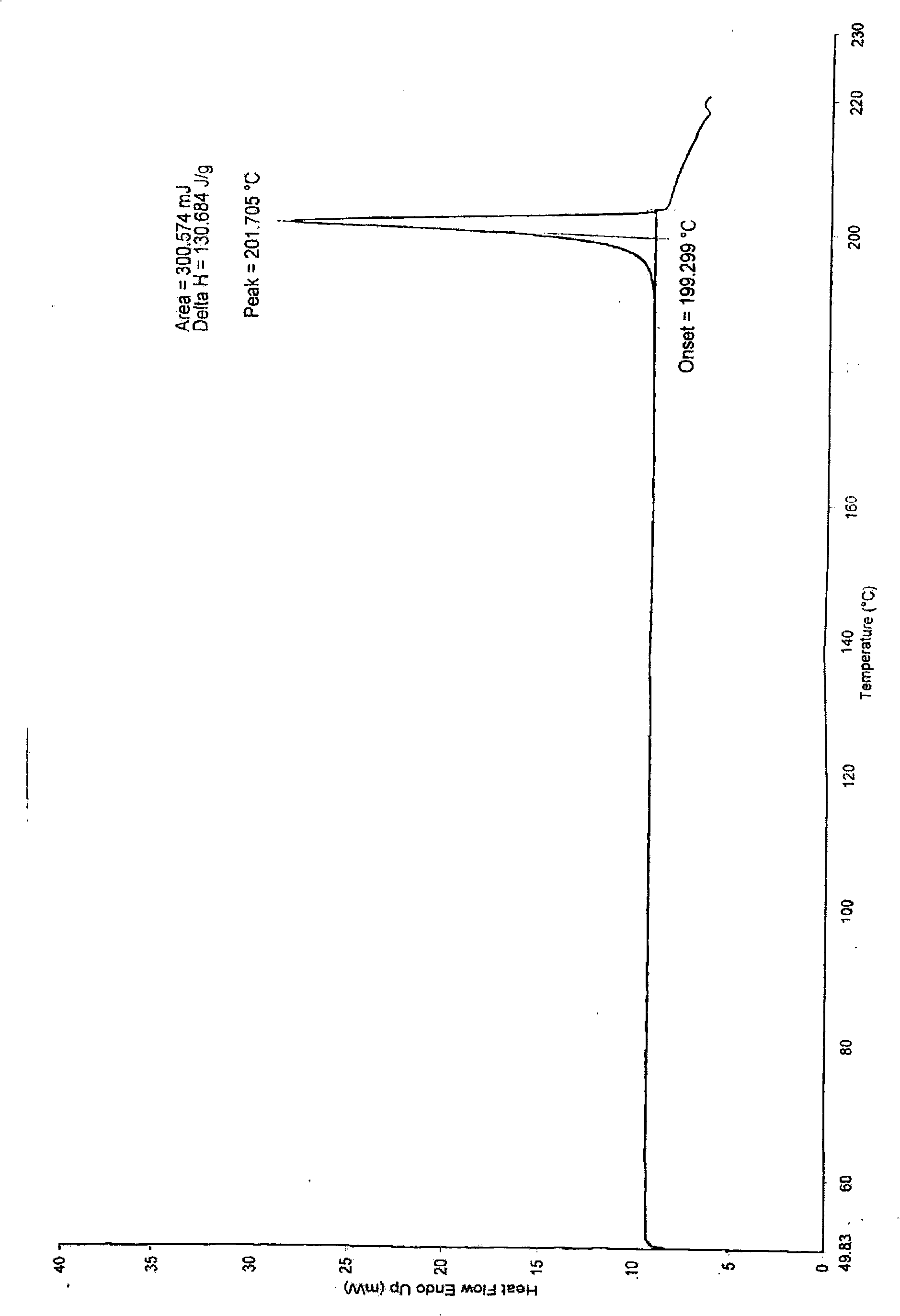
Synthesis and refinement of nelarabine
A technology of nerabine and a composition, which is applied to the refining treatment of nerabine and the field of preparation of nerabine, can solve the problems of low yield, increased cost, strict equipment requirements and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: Preparation of 6-O-methylguanosine
[0068] Take 248.0 grams of sodium methoxide and add it to 4.6 L of methanol, stir for 15 minutes to obtain a white turbid liquid, add 310 grams of 6-chloroguanosine material to dissolve quickly, and obtain a light yellow solution, and then precipitate a white solid, heat to reflux, and continue the reaction After 2.5 hours, cool to room temperature, carefully add hydrochloric acid solution dropwise under stirring to neutralize to PH=8-9, evaporate the solvent under reduced pressure, add 12L distilled water to the remaining light yellow viscous substance, dissolve it under stirring at 70°C, and then add 12g Activated carbon, hot filtration after 10min, filtrate, natural cooling and crystallization, filtration, to obtain a colorless crystalline solid, vacuum drying to obtain 196.7 g of 6-O-methylguanosine white solid, yield 64.3%, mp: 133-135 °C; TLC (ethyl acetate:methanol=6:1), product Rf=0.4, raw material Rf=0.45.
Embodiment 2
[0069] Embodiment 2: Preparation of 6-O-methylguanosine
[0070] Take 128 grams of sodium methoxide and add it to methanol, stir to obtain a white turbid liquid, add 160 grams of 6-chloroguanosine to dissolve rapidly, and obtain a light yellow solution, and then precipitate a white solid, heat to reflux, continue the reaction, and cool to At room temperature, carefully add hydrochloric acid solution dropwise under stirring to neutralize to PH = 8-9, evaporate the solvent, add distilled water to the remaining light yellow sticky substance, dissolve under stirring at 70°C, then add activated carbon, heat filter, filtrate, and cool naturally Crystallization and filtration gave a colorless crystalline solid, which was dried in vacuo to give 102.6 g of a white solid, ie, 6-O-methylguanosine. Yield 65.0%, mp: 133-135°C; TLC (ethyl acetate:methanol=6:1), product Rf=0.4, raw material Rf=0.45.
Embodiment 3
[0071] Embodiment 3: Preparation of 6-O-methylguanosine
[0072] Take 800 grams of sodium methoxide and add it to methanol, stir to obtain a white turbid liquid, add 1000 grams of 6-chloroguanosine to dissolve rapidly, and obtain a light yellow solution, and then precipitate a white solid, heat to reflux, continue the reaction, and cool to At room temperature, under stirring, carefully add hydrochloric acid solution dropwise to neutralize to PH = 8-9. After removing the solvent, add distilled water to the remaining light yellow viscous substance, dissolve it under stirring at 70°C, then add activated carbon, heat filter, filtrate, and naturally cool and analyze. crystal, filtered to obtain a colorless crystalline solid, and dried in vacuo to obtain 598.8 g of a white solid, namely 6-O-methylguanosine. Yield 60.7%, mp: 133-135°C; TLC (ethyl acetate:methanol=6:1), product Rf=0.4, raw material Rf=0.45.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com