Method for recycling lithium iron phosphate anode material from lithium ionic cell waste
A technology of lithium ion battery and lithium iron phosphate is applied in the field of recycling lithium iron phosphate cathode material, which can solve the problems of low capacity of lithium ion secondary battery, and achieve the effect of cost saving and high capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 10kg of lithium iron phosphate positive electrode waste slurry, put it into a material tray, put it into a high-temperature resistance furnace under a nitrogen atmosphere, bake it at a furnace temperature of 450°C for 5 hours, and then cool it with the furnace. Add the cooled material to the ferric nitrate ethanol solution whose concentration of ferric nitrate is 0.15mol / L and stir. Baking at 350° C. for 5 hours, and then cooling in the furnace to obtain the lithium iron phosphate cathode material.
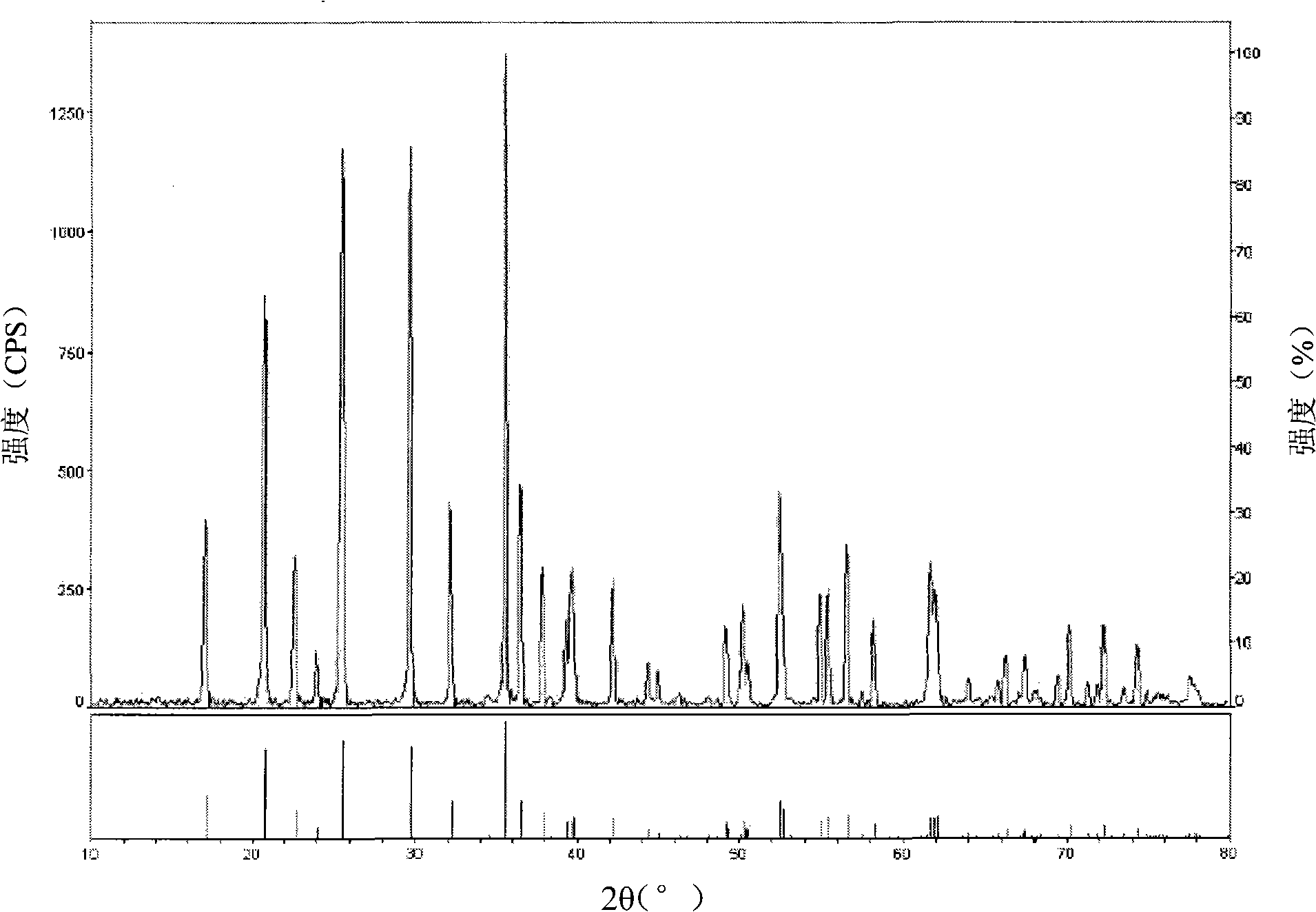
[0024] The product is tested with a Japanese Rigaku D / MAX2200PC X-ray diffractometer, and the obtained diffraction pattern is as follows figure 1 As shown, as can be seen from this figure, the diffraction peak tested in the upper part is basically the same as the diffraction peak of the standard lithium iron phosphate in the lower part, and the main peak peak intensity is higher, and the half-peak width (B value is 0.178) is narrower, indicating that Its crystals are ...
Embodiment 2
[0026] Take 10kg of lithium iron phosphate waste slurry, put it into a material tray, put it into a high-temperature resistance furnace under a nitrogen atmosphere, bake it at a furnace temperature of 450°C for 5 hours, and then cool it with the furnace. Add the cooled material to the ferric nitrate ethanol solution whose concentration of ferric nitrate is 0.2mol / L and stir. After the ethanol volatilizes, put it into a tray and put it into a high-temperature resistance furnace under a nitrogen atmosphere. Bake at 350°C for 5h, then cool with the furnace.
[0027] Then 5kg of the obtained material is added to the ball milling tank, and 70.6g of lithium carbonate and 109.4g of ammonium dihydrogen phosphate are weighed respectively according to 3% of the molar number of 5kg lithium iron phosphate, and all are added to the ball milling tank, and then alcohol and pickaxe are added. Balls, after ball milling for 2 hours, take out the evenly mixed material and dry it at 60°C. Finall...
Embodiment 3
[0030] Take 10 kg of lithium iron phosphate cathode sheets scrapped in production, put them into a material tray, put them into a high-temperature resistance furnace under a nitrogen atmosphere, bake them at a furnace temperature of 550°C for 4 hours, and then cool with the furnace. Put the baked pole piece on a sieve with a mesh of 5mm, knead the pole piece, and shake the sieve from time to time to completely separate the material and the current collector. Add the obtained powder into the ferric nitrate ethanol solution whose concentration of ferric nitrate is 0.1mol / L and stir. Bake at 400°C for 4 hours, then cool with the furnace.
[0031]Then 5kg of the obtained material is added to the ball milling tank, and 26g of lithium hydroxide monohydrate, 84.3g of diammonium hydrogen phosphate and 7.6g of acetylene black are weighed respectively according to 2% of the molar number of 5kg lithium iron phosphate, and all are added to the ball milling tank Then add alcohol and pick ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com