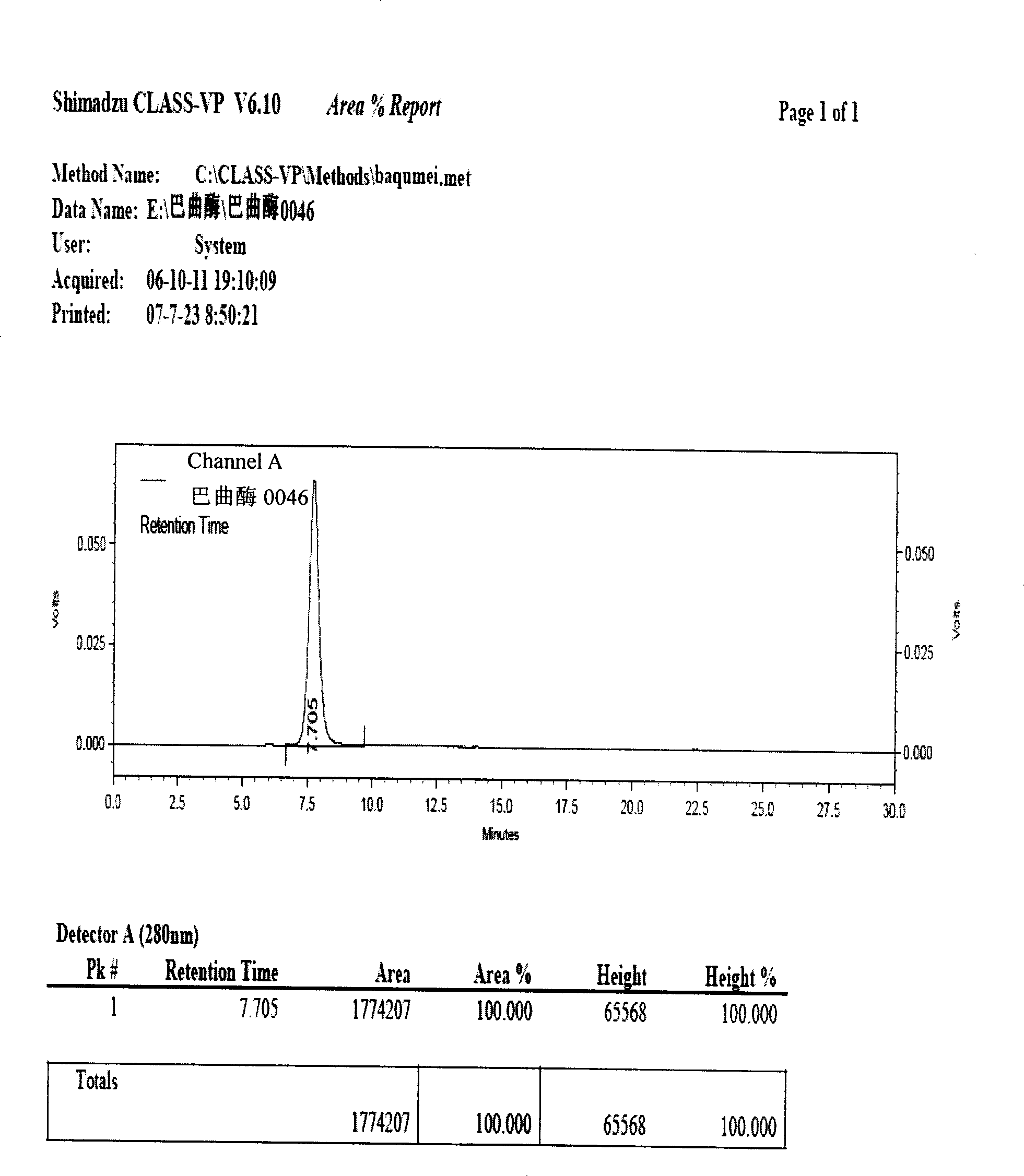
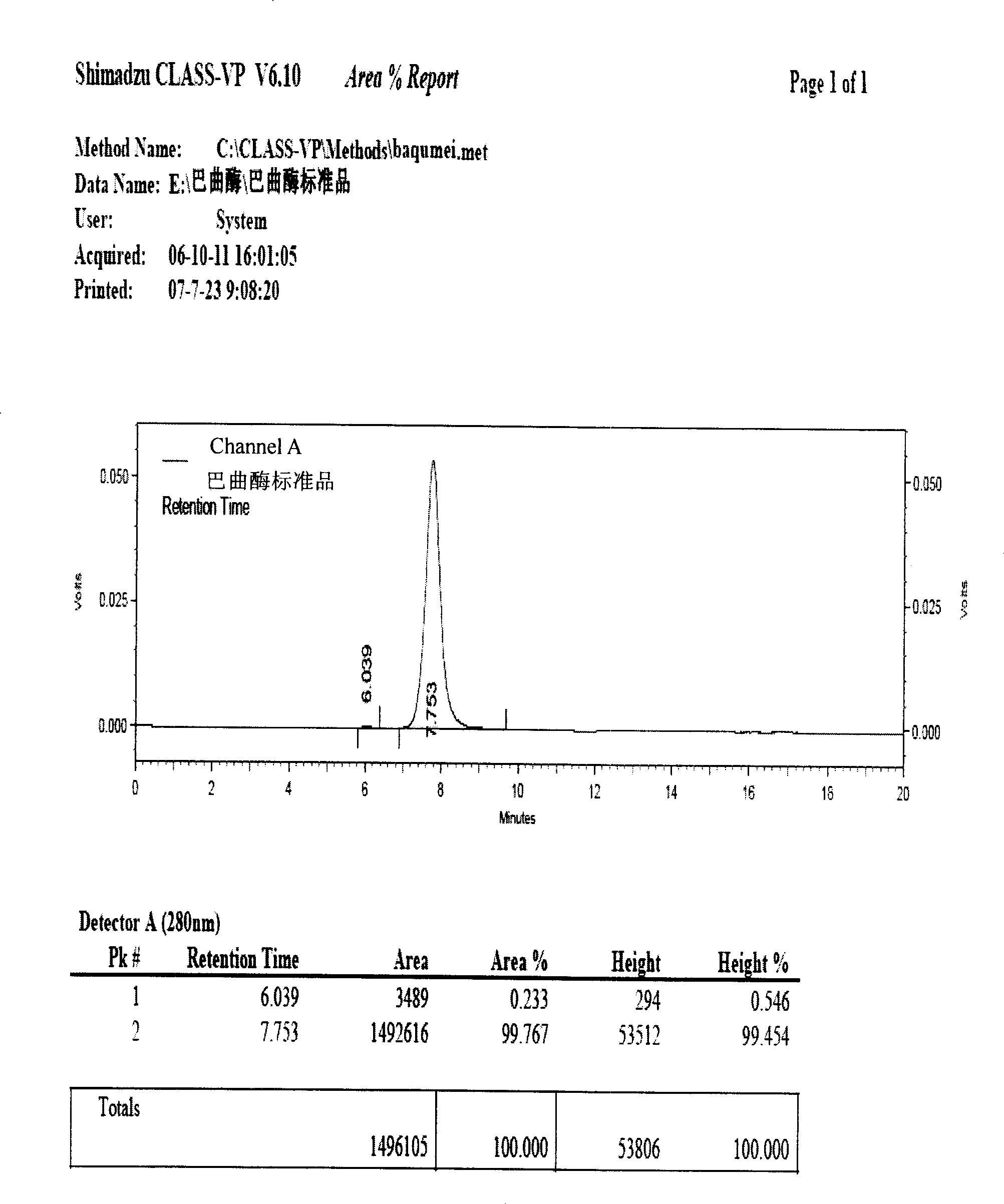
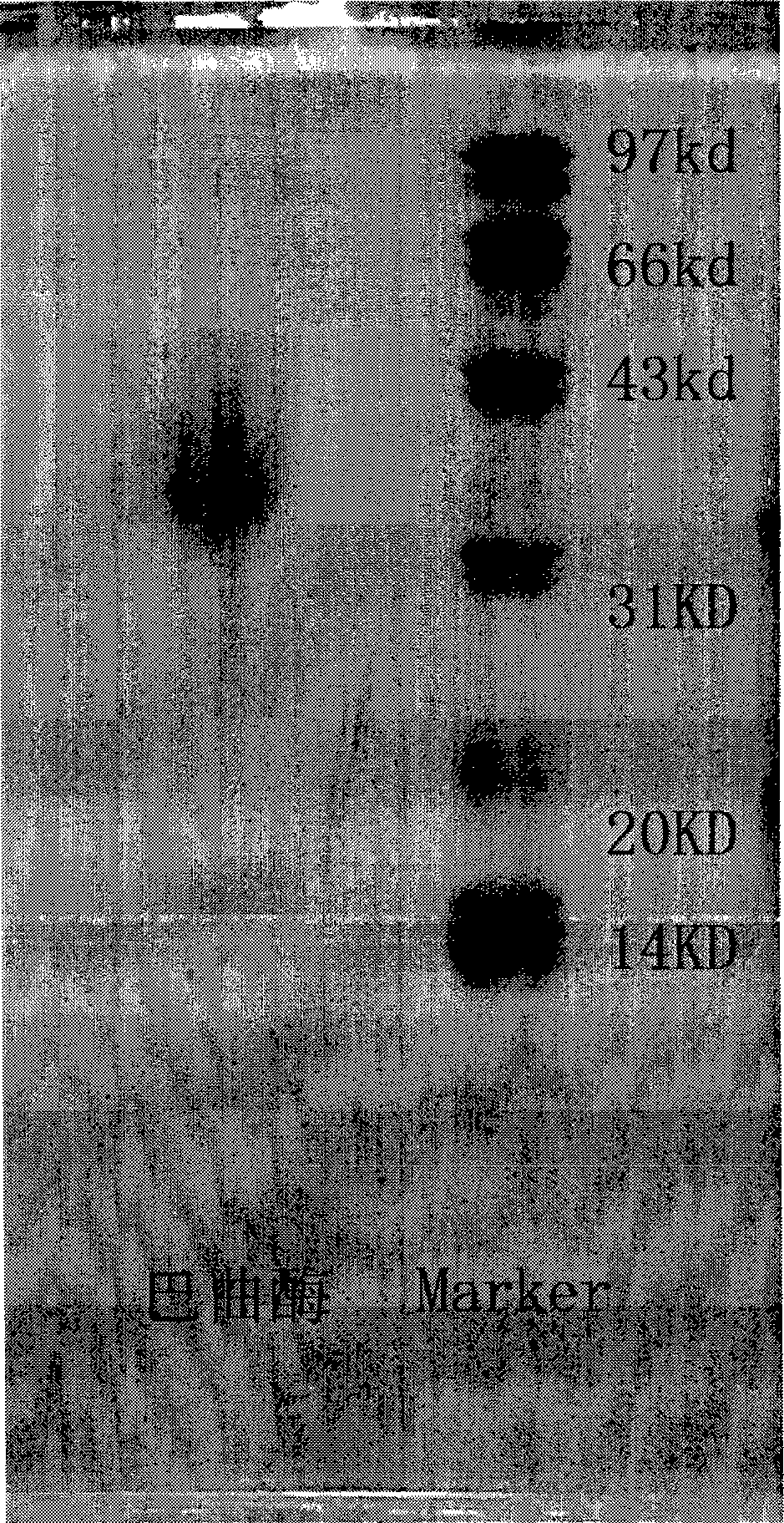Batroxobin freeze-dry powder injection and preparation method thereof
A technology of freeze-dried powder injection and batroxobin, which is applied in freeze-dried delivery, powder delivery, and pharmaceutical formulations. Achieve the effects of improving peripheral and microcirculation disorders, facilitating transportation and storage, and expanding the scope of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1. DEAE-Sepharose Fast Flow Chromatography
[0056] Weigh 6 g of Agkistrodon moojeni (Bothrops atrox moojeni, Sigma reagent catalog number: V5500), extract with 200 ml of 0.02mol / L pH7.4 Tris-HCl buffer for 4 hours, centrifuge at 4000rpm / min for 20 minutes, and take the supernatant ; Precipitate was extracted once again with 50ml of 0.02mol / L pH7.4 Tris-HCl buffer in the same way, and the supernatants were combined, and the pH was adjusted to 7.4 to obtain the extract. Put the extract on a well-balanced DEAE-Sepharose Fast Flow chromatography column, first use 0.02mol / L Tris-HCl buffer to elute unadsorbed impurities, and then use a buffer solution containing 0-0.6mol / L NaCl for gradient washing Remove, collect batroxobin activity peak, obtain active component collection solution A 300ml.
[0057] 2. Affinity chromatography
[0058] Put the above-mentioned active component collection solution A on a well-balanced affinity chromatography column, and first use 1.0mol / L N...
Embodiment 2
[0069] Get batroxobin crude drug (prepared by embodiment 1, the same below) 10000BU, it is added to dissolve in 5mmol / L citrate buffer (pH5.0) 1000ml, obtains solution, adds sucrose in solution 1g, 80g of mannitol, stirred and dissolved and shaken evenly, sterilized and filtered, the filtrate was aseptically divided into 2000 tubes, each containing 5BU, freeze-dried to obtain batroxobin freeze-dried powder preparation.
[0070] The preparation was placed at room temperature for 2 years, and the titer was determined to be 4.5 BU.
Embodiment 3
[0072] Take batroxobin crude drug 10000BU, add it to 1000ml of 10mmol / L citrate buffer solution (pH5.4) and dissolve it to obtain a solution, add 0.5g of glycine and 50g of dextran to the solution, stir to dissolve and shake well , sterilized and filtered, and the filtrate was aseptically divided into 2000 tubes, each with a content of 5 BU, and freeze-dried to obtain batroxobin freeze-dried powder preparation.
[0073] The preparation was placed at room temperature for 2 years, and the titer was determined to be 4.4BU.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com