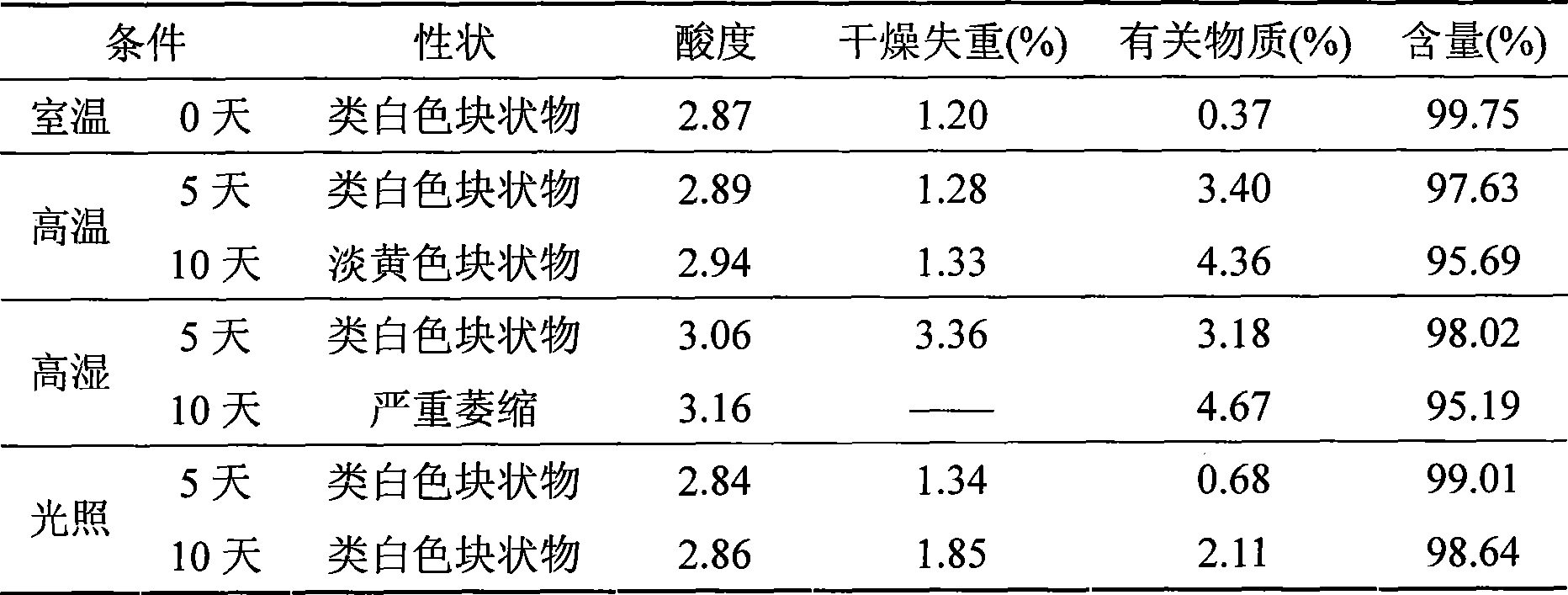
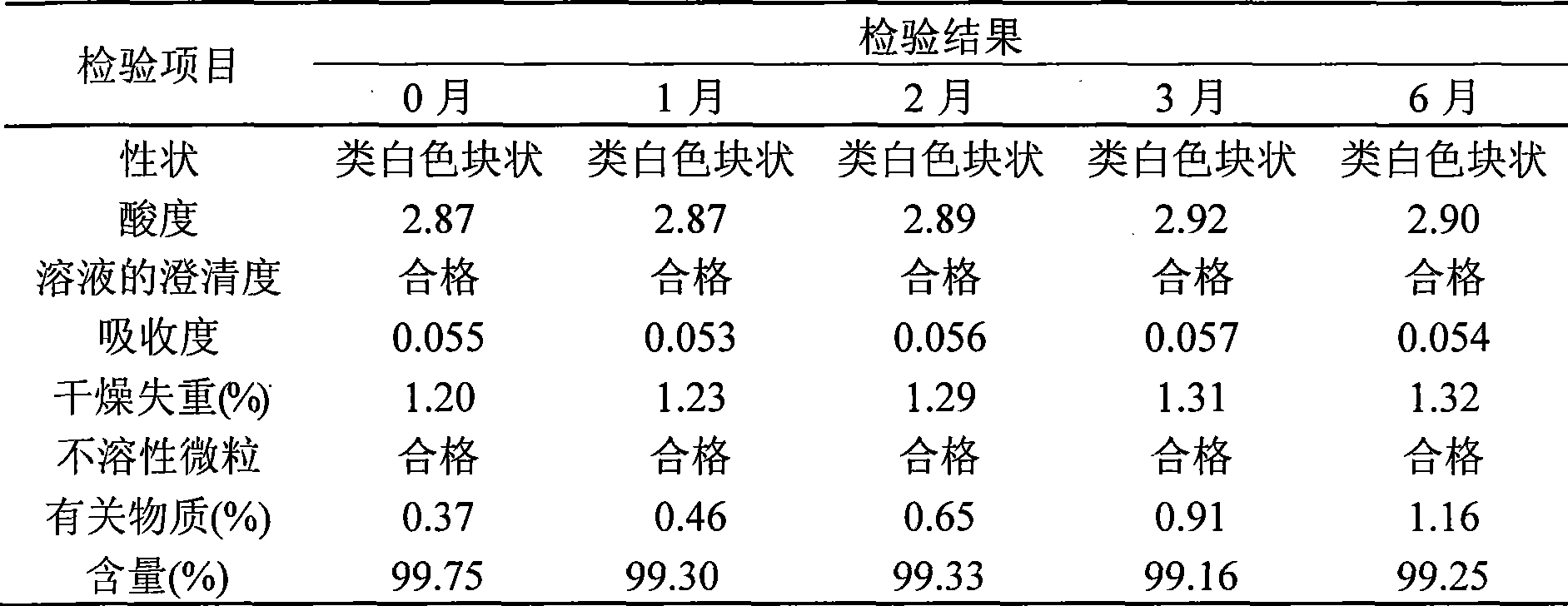
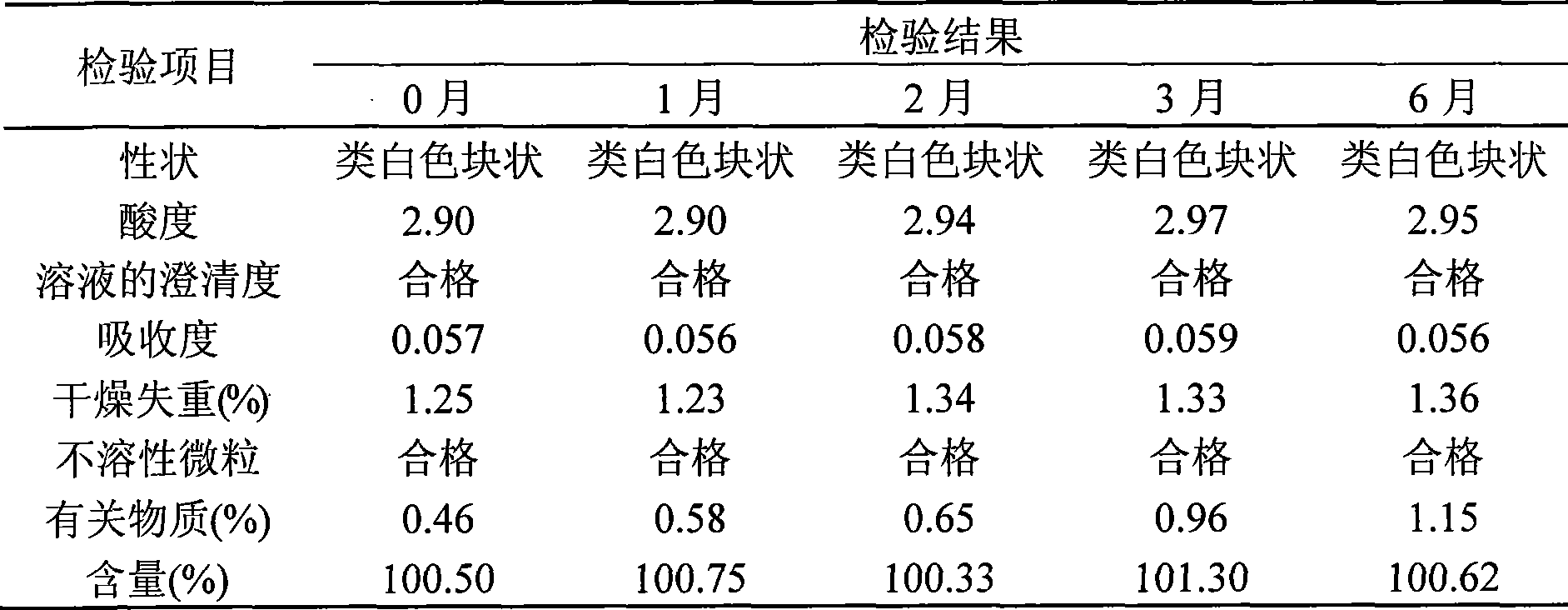
Thiamphenicol aminoacetate hydrochloride sterile freeze-drying preparation for injection and preparation method thereof
A technology of thiamphenicol hydrochloride and thiamphenicol acid, which is applied in the field of sterile freeze-dried preparations of thiamphenicol hydrochloride glycine ester for injection and its preparation, can solve the problems of unfavorable drug stability, damage, unfavorable drug carrying and transportation And other problems, to achieve the effect of good stability, long service life, convenient transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The sterile freeze-dried preparation of thiamphenicol hydrochloride glycinate for injection is prepared from 1.3kg of thiamphenicol hydrochloride glycinate, 300ml of PEKG-400, 300ml of propylene glycol and adding water for injection to 6000ml. Make 1000 bottles, each bottle of freeze-dried preparation contains thiamphenicol hydrochloride glycinate 1.3g (calculated as thiamphenicol 1g)
[0019] The preparation method of thiamphenicol hydrochloride glycinate sterile freeze-dried preparation for injection is:
[0020] (1) Get 1.3kg of thiamphenicol hydrochloride glycine ester (calculated as thiamphenicol 1g) of the prescription amount, add 60% water for injection of the total amount, stir to fully disperse, add 300ml of the prescription amount while stirring PEKG-400 and 300ml propylene glycol, stirred in a 40°C water bath until completely dissolved.
[0021] (2) Add activated carbon for needles according to 0.1% of the solution volume, stir for 30 minutes, filter, add wa...
Embodiment 2
[0025] The sterile freeze-dried preparation of thiamphenicol hydrochloride glycinate for injection is prepared from 0.6 kg of thiamphenicol hydrochloride glycinate, 0.1 kg of mannitol, 100 ml of propylene glycol and adding water for injection to 2000 ml. Make 1000 bottles, each bottle of lyophilized preparation contains 0.6g of thiamphenicol hydrochloride glycinate (0.5g in terms of thiamphenicol)
[0026] The preparation method of thiamphenicol hydrochloride glycinate sterile freeze-dried preparation for injection is:
[0027] (1) Get 0.6kg of thiamphenicol hydrochloride glycine ester, add 70% water for injection in total, stir to fully disperse, add 0.1kg of mannitol and 100ml of propylene glycol in the prescribed amount while stirring, stir in a 50°C water bath until completely dissolved.
[0028] (2) Add activated carbon for needles according to 0.2% of the solution volume, stir for 40 minutes, filter, add water for injection to 2000ml, filter with a 0.22μm microporous mem...
Embodiment 3
[0032] The sterile freeze-dried preparation of thiamphenicol hydrochloride glycinate for injection is prepared from 0.6 kg of thiamphenicol hydrochloride glycinate, 0.2 kg of mannitol and adding water for injection to 5000 ml. Make 1000 bottles;
[0033] Each bottle of lyophilized preparation contains 0.6 g of thiamphenicol hydrochloride glycine ester (0.5 g as thiamphenicol).
[0034] The preparation method of thiamphenicol hydrochloride glycinate sterile freeze-dried preparation for injection is:
[0035] (1) Get 0.6kg of thiamphenicol hydrochloride glycine ester of prescription quantity, add the water for injection of 70% of total amount, stir to fully disperse, add 0.2kg of mannitol while stirring, stir in 30 ℃ of water baths until fully dissolving.
[0036] (2) Add activated carbon for needles according to 0.05% of the solution volume, stir for 30 minutes, filter, add water for injection to 5000ml, filter with a 0.22μm microporous membrane, measure the content, fill in c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com