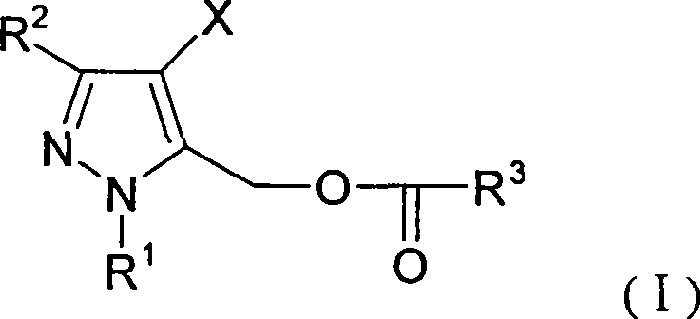
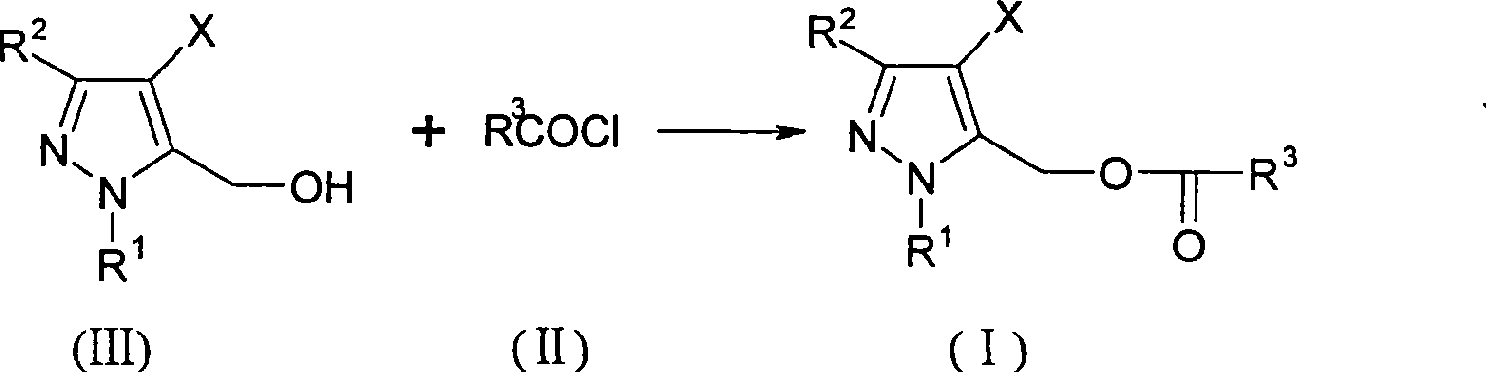
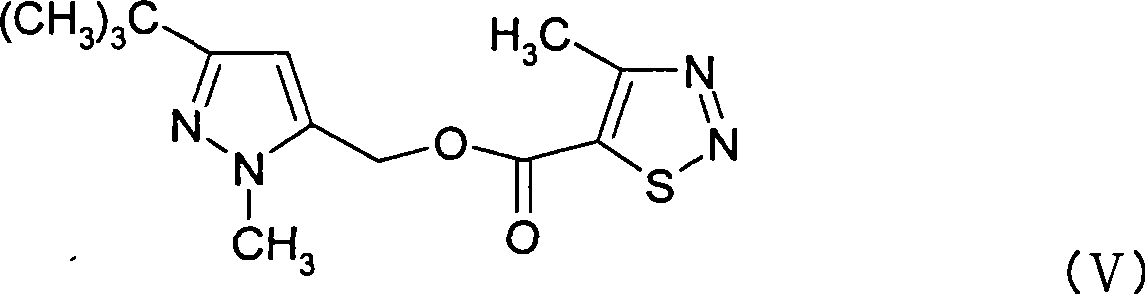
Pyrazole methanol esters compounds, preparation method and application thereof
A technology of ester compounds and pyrazole carbinol, which is applied in the field of pesticides and can solve the problems that the application of pyrazole carbinol ester compounds has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the preparation of (4-chloro-3-ethyl-1-methyl-5-pyrazolyl) methyl p-tert-butylbenzoate
[0055] A solution composed of p-tert-butylbenzoyl chloride (10.9g, 0.055mol) and toluene (30ml) was slowly added dropwise under stirring and cooling in an ice-water bath to 4-chloro-3-ethyl-1-methyl- In a mixture of 5-hydroxymethylpyrazole (8.7g, 0.05mol), triethylamine (6.0g, 0.06mol) and toluene (200ml). After the dropwise addition, the temperature of the reaction mixture was raised to about 30° C., and the stirring was continued at this temperature. It took about 4-5 hours to follow the chromatographic tracking until the reactant was basically converted completely. The resulting reaction mixture was poured into water (300ml). Let stand to layer. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and precipitated under reduced pressure. The residue was the title compound (Compound No.8 in Table 1), weighing 15 g, and was a li...
Embodiment 2
[0058]According to the method described in Example 1, Compound No.1 was prepared by reacting p-tert-butylbenzoyl chloride with 3-ethyl-1-methyl-5-hydroxymethylpyrazole, using benzoyl chloride and 3-ethyl Compound No.2 was prepared by reacting p-chlorobenzoyl chloride with 3-ethyl-1-methyl-5-hydroxymethylpyrazole to prepare compound No. 3. Compound No.4 was prepared by reacting o-methoxybenzoyl chloride with 3-ethyl-1-methyl-5-hydroxymethylpyrazole, using 2-chloro-5-nitro-benzoyl chloride and 3- Compound No.5 was prepared by the reaction of ethyl-1-methyl-5-hydroxymethylpyrazole, using Z-cis-3-(2-chloro-3,3,3-trifluoro-1-propenyl)- 2,2-dimethylcyclopropanyl formyl chloride and 3-ethyl-1-methyl-5-hydroxymethylpyrazole react to prepare compound No.6, using 2,2-dimethyl-3-(2 , 2-dichlorovinyl) cyclopropanyl formyl chloride and 3-ethyl-1-methyl-5-hydroxymethylpyrazole reaction to prepare compound No.7, using p-chloromethylbenzene to chlorinated benzoyl chloride Compound No.9 was ...
preparation Embodiment 1
[0068] Formulation Example 1: wettable powder
[0069] 20 parts of the compound of the present invention, 10 parts of white carbon black, 55 parts of kaolin, 10 parts of sodium lauryl sulfate and 5 parts of calcium lignosulfonate were uniformly mixed together and jet milled to obtain a wettable powder with an active ingredient of 20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com