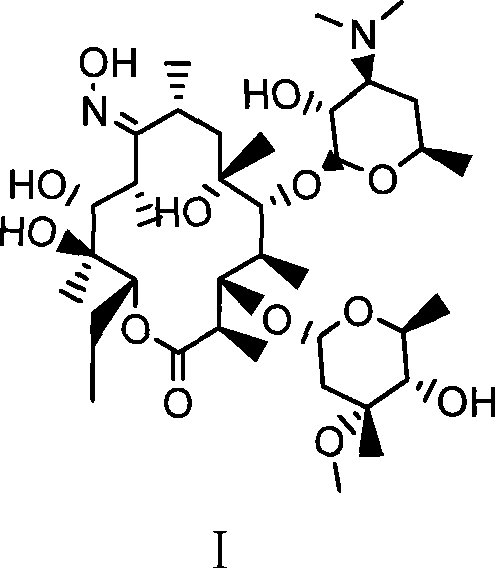
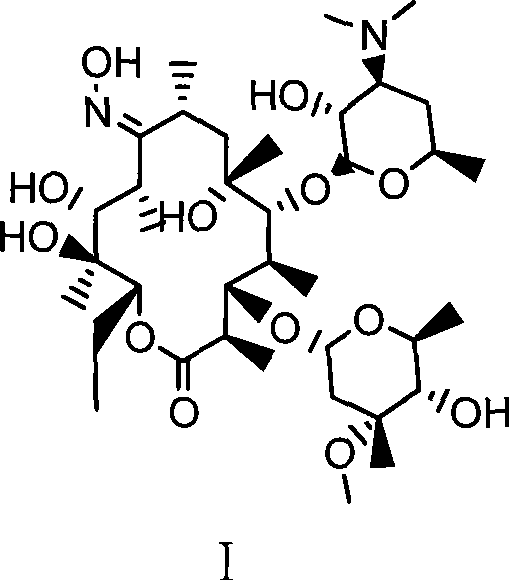
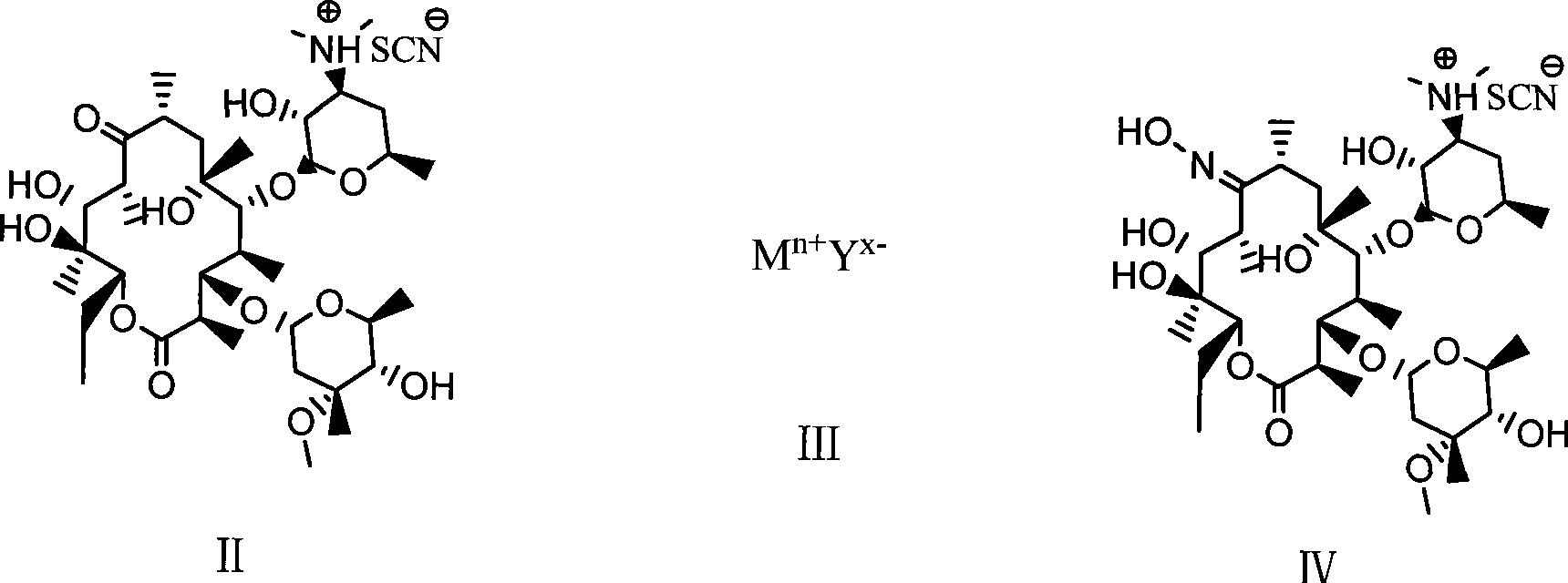Preparation method of erythromycin A(E) oxime
A technology of erythromycin and erythromycin thiocyanate, applied in sugar derivatives, organic chemistry, etc., can solve the problems of low E/Z ratio of erythromycin oxime, unsuitable for large-scale production, poor product quality, etc. , to achieve the effect of low raw material cost, good quality and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. A preparation method of erythromycin A(E) oxime, followed by the following steps:
[0025] 1) First add 140ml methanol, hydroxylamine hydrochloride (42.3g, 0.61mol) and diammonium hydrogen phosphate (81.1g, 0.61mol) in a 500ml four-necked flask, and react for 30min at room temperature (generally 0-40°C). Then, erythromycin thiocyanate (65g, 0.087mol) was added, and the temperature was raised to 60°C to react for 32 hours. Then, the above reaction product was cooled to 40°C and 220 ml of water was added for crystallization. After adding water, cool to 20°C and filter, and after washing with 100ml*3 water three times, 55.2g of erythromycin A(E) oxime thiocyanate is obtained. HPLC: erythromycin A(E) oxime 97.5%, erythromycin A(z) oxime 0.9%.
[0026] 2) Add 55.0g (0.068mol) of erythromycin A(E) oxime thiocyanate and 170ml of methanol in a 500ml four-necked flask. After cooling to 20℃, slowly add sodium hydroxide with a mass concentration of 25%. 21.8g (0.136mol) of ...
Embodiment 2
[0027] Example 2. A preparation method of erythromycin A(E) oxime, followed by the following steps:
[0028] 1) First, add 70ml isopropanol, hydroxylamine hydrochloride (48.4g, 0.696mol) and potassium bicarbonate (69.6g, 0.696mol) in a 500ml four-necked flask, and react for 30min at room temperature. Then erythromycin thiocyanate (65g, 0.087mol) was added and reacted at 40°C for 80h. Then, the above reaction product was cooled to 38°C and 220 ml of water was added for crystallization. After adding water, cool to 20°C and filter. After washing with 100ml*3 water three times, 53.6g of erythromycin A(E) oxime thiocyanate is obtained. HPLC is: erythromycin A(E) oxime 93.5%, erythromycin A(z) oxime 2.0%.
[0029] 2) Add 55.0g (0.068mol) of erythromycin A(E) oxime thiocyanate and 170ml of ethanol in a 500ml four-necked flask. After cooling to 20℃, slowly add 25% sodium hydroxide dropwise. Aqueous solution 30.0g (0.187mol); after dripping, stirring and reacting for 22h; then 270ml of wat...
Embodiment 3
[0030] Example 3. A preparation method of erythromycin A(E) oxime, followed by the following steps:
[0031] 1) First, add 90 ml of ethanol, hydroxylamine hydrochloride (18.1 g, 0.26 mol) and sodium monohydrogen phosphate (36.9 g, 0.26 mol) to a 500 ml four-necked flask, and react for 30 min at room temperature. Then, erythromycin thiocyanate (65g, 0.087mol) was added, and the temperature was raised to 80°C to react for 24h. Then, the above reaction product was cooled to 40°C and 220 ml of water was added for crystallization. After adding water, cool to 20°C and filter. After washing with 100ml*3 water three times, 43.8g of erythromycin A(E) oxime thiocyanate is obtained. HPLC: erythromycin A(E) oxime 94.8%, erythromycin A(z) oxime 1.8%.
[0032]2). Add 40.4g (0.05mol) of erythromycin A(E) oxime thiocyanate and 100ml of isopropanol in a 500ml four-necked flask. After cooling to 5℃, slowly add hydrogen with a mass concentration of 25%. 10.0 g (0.062 mol) of sodium oxide aqueous sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com