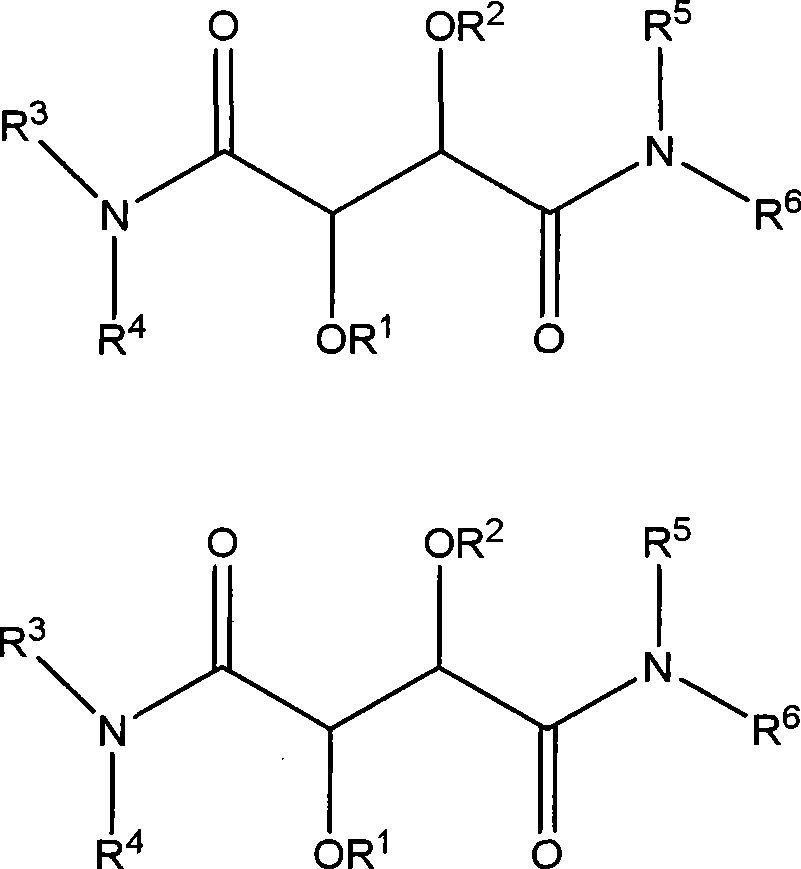
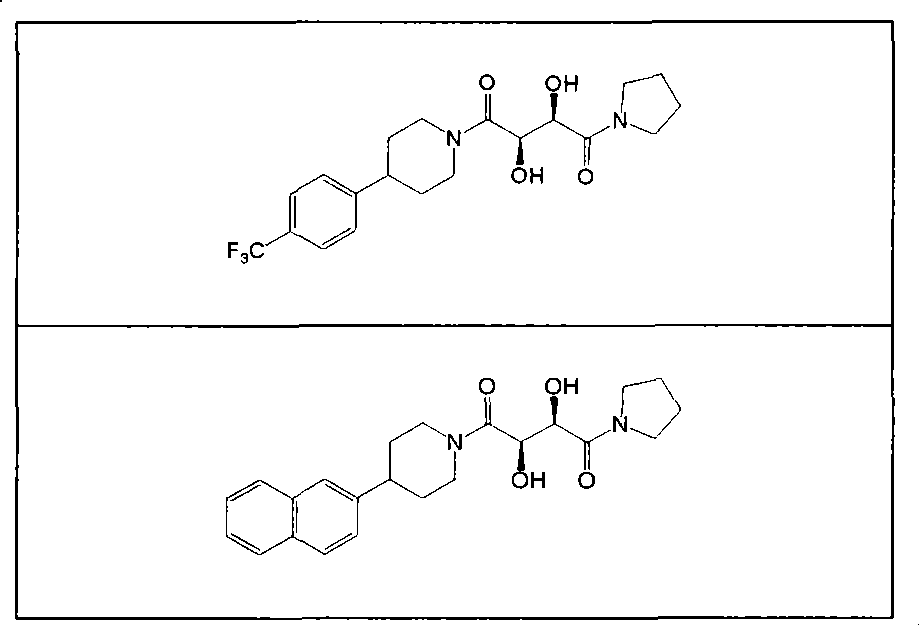
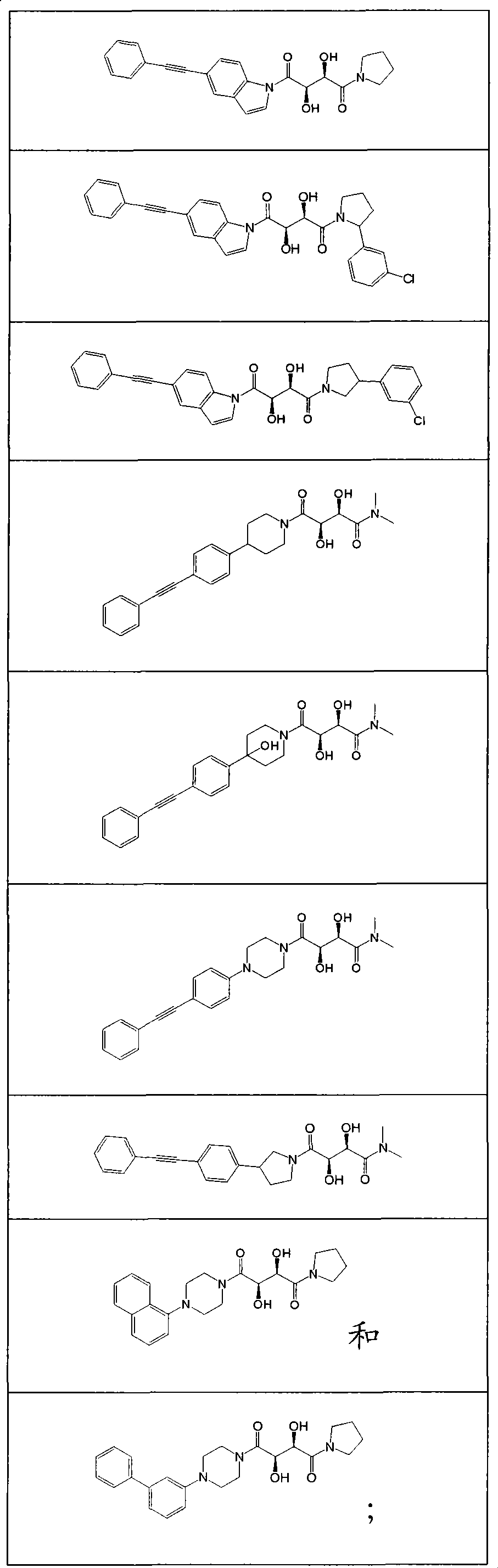
Compounds for the treatment of inflammatory disorders and microbial diseases
A compound and disease technology, applied in allergic diseases, metabolic diseases, drug combinations, etc., can solve problems such as lack of mechanical properties of cartilage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0310]
[0311] Part A:
[0312] To a stirred solution of dimethyl L-tartrate (1) (29.8 g, 136 mmol) in methanol (60 mL) over 30 minutes was added potassium hydroxide (6.9 g, 123 mmol) in water at 0°C (ice bath) (20mL). The reaction mixture was stirred at room temperature for 3 hours. The volatiles were removed in vacuo, water (40 mL) was added, and the alkaline solution was washed with ether (30 mL x 3). The alkaline solution was acidified to pH 2.0 with 6N HCl, saturated with solid sodium chloride, and the product was extracted into ether (40 mL x 4). It was dried over magnesium sulfate and concentrated to obtain compound 2 (22.2 g, 79% yield) as a colorless oil.
[0313] Part B:
[0314] To compound 2 (1.13g, 5.53mmol) and O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU) (3.2 g, 8.3 mmol) To the mixture in DMF (20 mL) was added amine building block (1.2 equivalents) and diisopropylethylamine (2.89 mL, 16.59 mmol). The reaction mixture was sti...
Embodiment 2A
[0321]
[0322] Part A:
[0323] To 4-iodoaniline (8) (440mg, 2mmol), copper iodide (7.6mg, 0.04mmol) and dichlorobis (triphenylphosphine) palladium (II) (14mg, 0.02mmol) in THF (5mL) Phenylacetylene (244.8 mg, 2.4 mmol) and triethylamine (556 μL, 4 mmol) were added to the mixture. The reactor was purged with argon, and the reaction mixture was stirred at room temperature for 16 hours. LC-MS analysis of the reaction showed that the reaction was complete. Ethyl acetate (5 mL) was added, and the precipitate was removed by passing through a celite column. The filtrate was concentrated, and the crude product was purified by flash column chromatography (SiO 2 , 6% ethyl acetate / hexane) to obtain compound 9 as a brown solid (321 mg, 82% yield). HPLC-MSt R =1.88min(UV 254nm ); The following formula quality calculation value C 14 H 11 N 193.1, found LCMS m / z 194.1 (M+H).
Embodiment 2B
[0325]
[0326] Part A:
[0327] Compound 11 was prepared from 5-iodoindole (10) using the Sonogashira coupling conditions described in Example 2A, Part A. HPLC-MS t R =2.06min(UV 254nm ); The following formula quality calculation value C 16 H 11 N 217.1, found LCMS m / z 218.1 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com