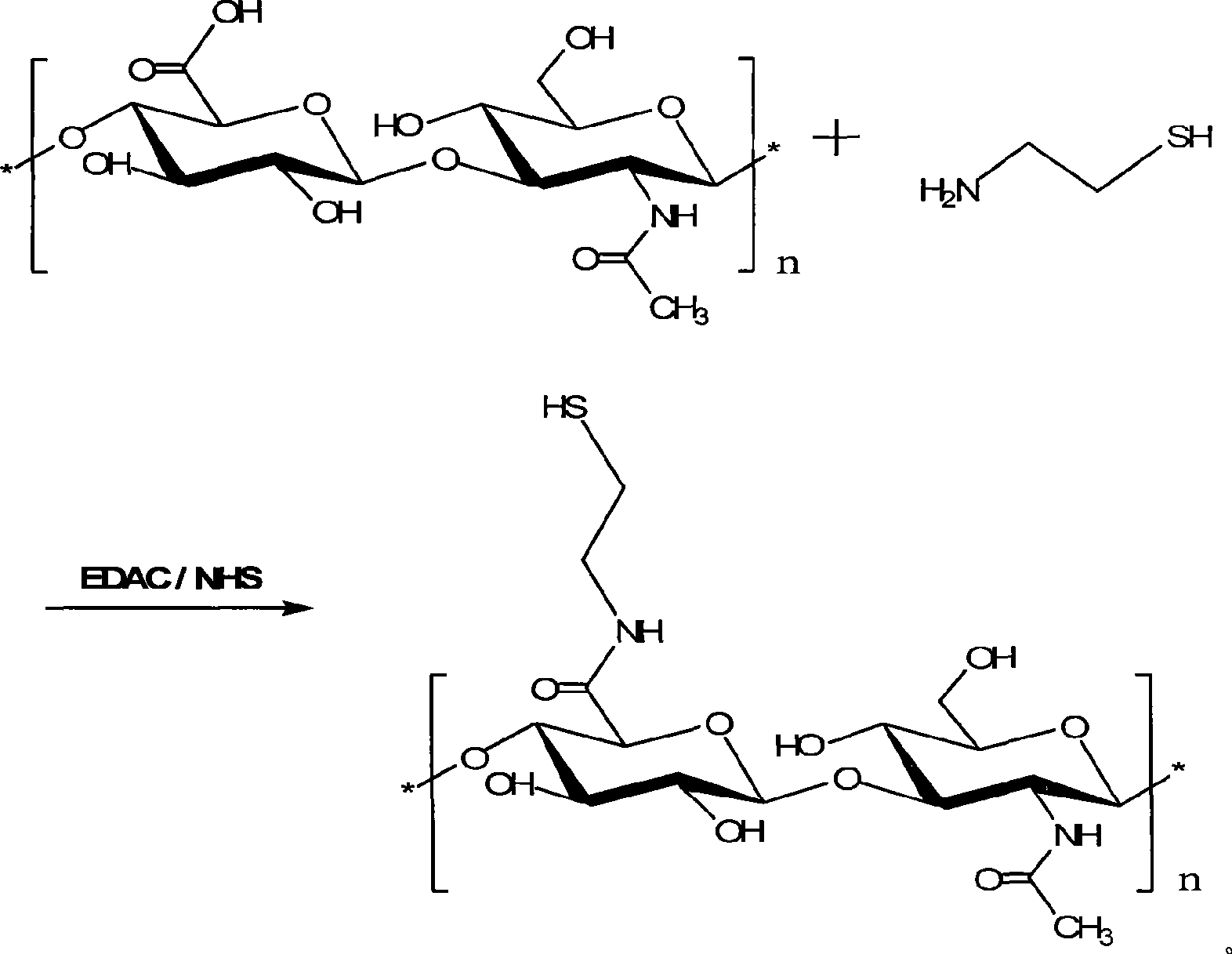
Cysteamine modified sulfhydryl hyaluronic acid couplet, preparation and application thereof
A technology of hyaluronic acid and cysteamine hydrochloride is applied in biochemical equipment and methods, medical preparations with non-active ingredients, microorganisms, etc., and can solve the problems of low content of disulfide bonds and poor in-situ gelation. , to achieve the effect of increasing local concentration, improving mechanical properties and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 200mg molecular weight is 5×10 5 Sodium hyaluronate of Da was dissolved in 50ml deionized water to obtain a 0.4% hyaluronic acid solution, and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide was added with a final concentration of 0.96% respectively Hydrochloride (EDAC) and 0.58% N-hydroxysuccinimide (NHS), adjusted to pH 5.5 after dissolving, stirred for 45 minutes; 400 mg of semicarpine hydrochloride was added to adjust the pH of the reaction solution to 4.75, and reacted in the dark for 5 hours. After the reaction, the reaction mixture was placed in a dialysis bag with a cut-off molecular weight of 3500Da, dialyzed with HCl solution (pH 4), 1% NaCl-containing HCl solution (pH 4), HCl solution (pH 4) successively, freeze-dried to obtain cysteamine Modified thiolated hyaluronic acid conjugates.
Embodiment 2
[0033] The molecular weight of 300mg is 1.98×10 4 Da sodium hyaluronate was dissolved in 50ml of deionized water to obtain a 0.6% hyaluronic acid solution, and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide was added with a final concentration of 0.19% respectively Hydrochloride (EDAC) and 0.12% N-hydroxysuccinimide (NHS) were dissolved, adjusted to pH 5, and stirred for 30 minutes; 300 mg of sphenamine hydrochloride was added to adjust the pH of the reaction solution to 3, and the reaction was protected from light for 4 hours. After the reaction, the reaction mixture was placed in a dialysis bag with a cut-off molecular weight of 3500Da, dialyzed with HCl solution (pH 4), 1% NaCl-containing HCl solution (pH 4), HCl solution (pH 4) successively, freeze-dried to obtain cysteamine Modified thiolated hyaluronic acid conjugates.
Embodiment 3
[0035] The molecular weight of 400mg is 1×10 5 Sodium hyaluronate of Da was dissolved in 50ml deionized water to obtain a 0.8% hyaluronic acid solution, and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide was added with a final concentration of 0.48% respectively Hydrochloride (EDAC) and 0.29% N-hydroxysuccinimide (NHS) were dissolved, adjusted to pH 5.5, and stirred for 40 minutes; 200 mg of sphenamine hydrochloride was added to adjust the pH of the reaction solution to 4, and reacted in the dark for 5 hours. After the reaction, the reaction mixture was placed in a dialysis bag with a cut-off molecular weight of 3500Da, dialyzed with HCl solution (pH 4), 1% NaCl-containing HCl solution (pH 4), HCl solution (pH 4) successively, freeze-dried to obtain cysteamine Modified thiolated hyaluronic acid conjugates.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com