Method for producing sulfurated hydrogen and calcium salt with calcium sulphide
A calcium sulfide and hydrogen sulfide technology, applied in chemical instruments and methods, hydrogen sulfide, inorganic chemistry, etc., can solve the problems of discarding and stacking, difficult to use, etc., and achieve easy control of the production process, complete process flow, and high reaction conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
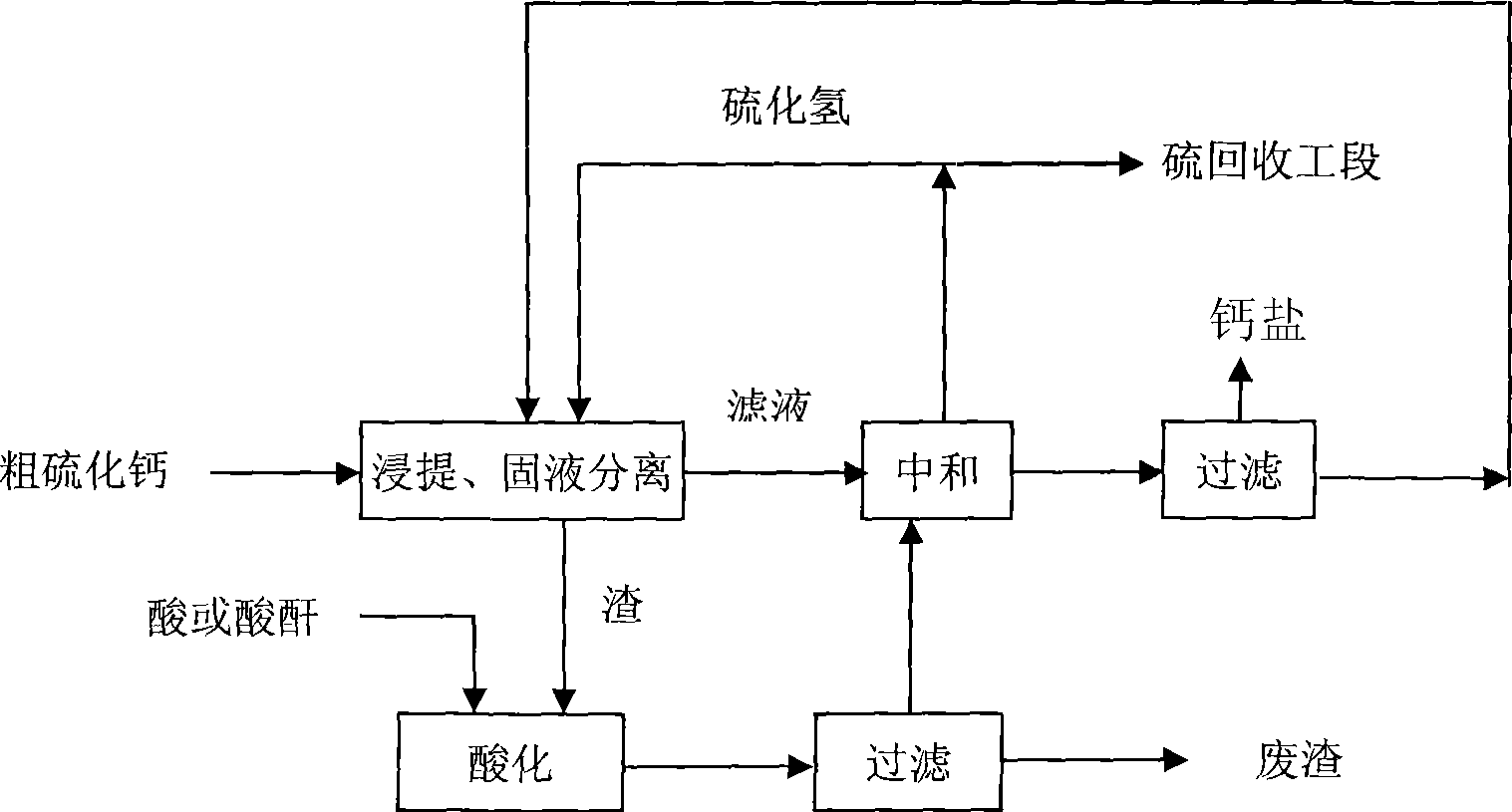
[0020] Raw material 1: Coarse calcium sulfide CaS = 52.3%; moisture ≤ 1%; particle size 25 mesh sieve residue ≤ 3%
[0021] Raw material 2: wet-process phosphoric acid after defluorination and desulfurization treatment
[0022] P 2 o 5 % CaO% Fe 2 o 3 % MgO% Al 2 o 3 % SO 3 % F% 49.55 0.25 0.93 0.8 2.06 0.050 0.23
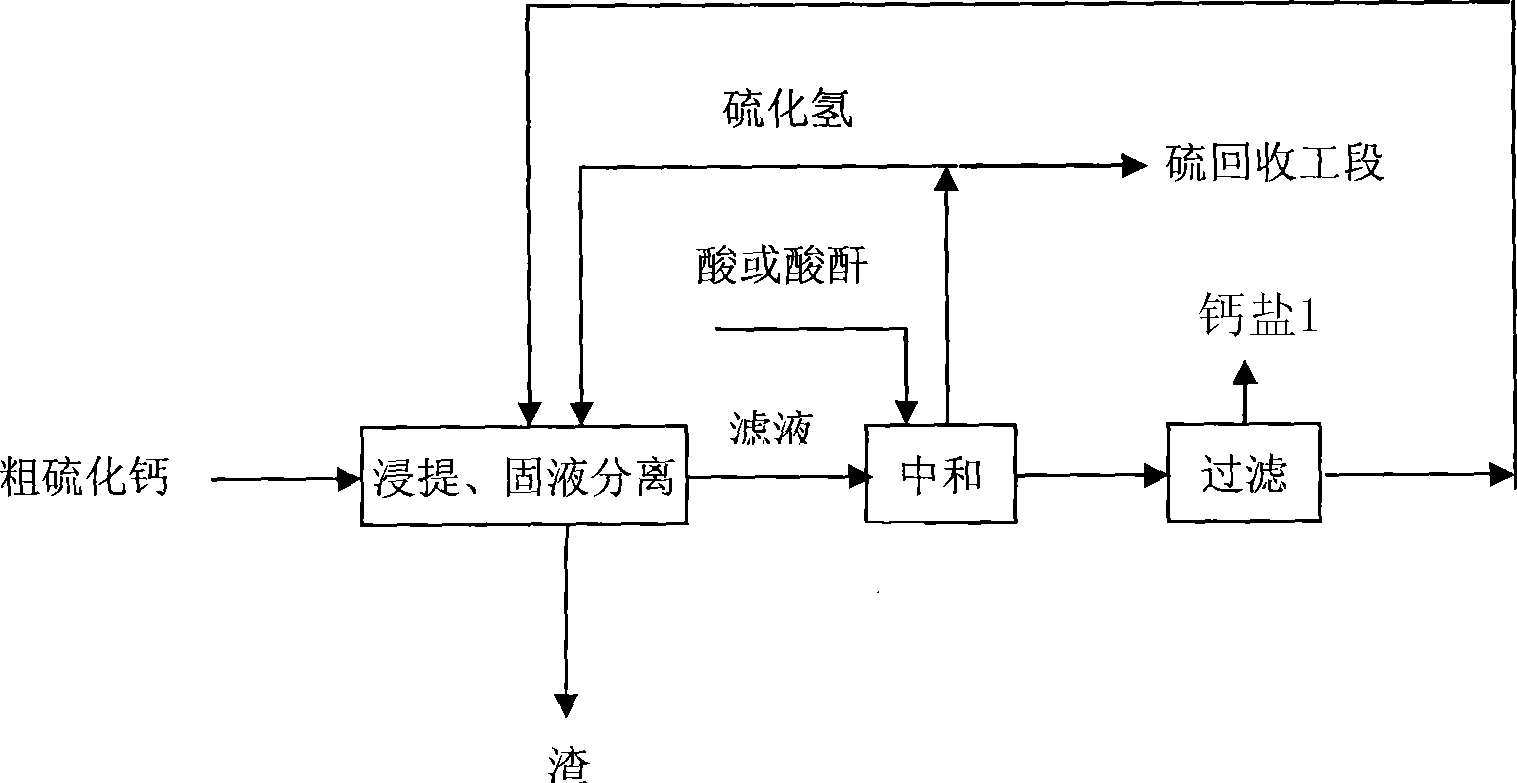
[0023] Add crude calcium sulfide to water, stir to form a suspension, and then lead to hydrogen sulfide reaction to obtain the intermediate product calcium hydrosulfide, which is separated by hydrocyclone to obtain calcium hydrosulfide clear liquid and slurry, and add the slurry to defluorinated wet-process phosphoric acid Medium reaction, the hydrogen sulfide gas is collected through the gas phase outlet, and after filtration and separation to remove slag, the clear liquid is reacted with the calcium hydrosulfide aqueous solution obtained by the previous hydrocyclone separation, and the hydrogen sulfide gas is collected...
Embodiment 2
[0026] Raw material is with embodiment 1.
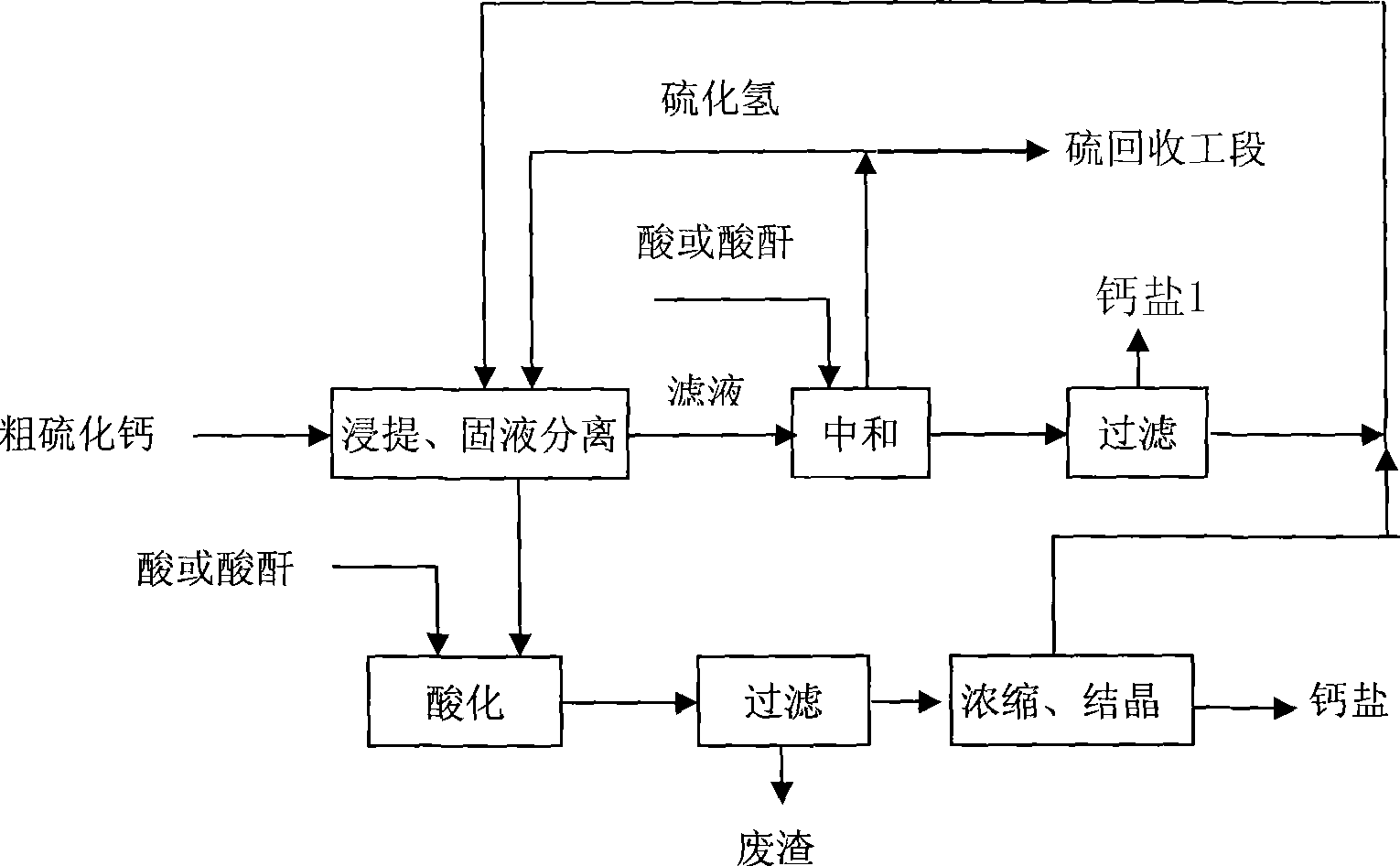
[0027] Add crude calcium sulfide into water, stir to form a suspension, then pass through hydrogen sulfide to react to obtain intermediate product calcium hydrosulfide, and filter to obtain calcium hydrosulfide supernatant. Add the calcium hydrosulfide aqueous solution to the defluorinated wet-process phosphoric acid, and the hydrogen sulfide gas generated by the reaction is collected through the gas phase outlet; when the pH value of the solution reaches 2.8-3.2, the reaction is terminated. The solution is concentrated and crystallized to obtain a crude product of calcium dihydrogen phosphate, and then washed and dried to obtain a product of calcium dihydrogen phosphate. The obtained calcium dihydrogen phosphate meets the national feedstuff calcium dihydrogen phosphate product standard, and the product quality is as follows:
[0028] P Ca F Pb As 22.4 16.3 0.13 0.001 0.001
Embodiment 3
[0030] Raw material is with embodiment 1.
[0031] Add crude calcium sulfide to water, stir to form a suspension, and then introduce hydrogen sulfide to react to obtain intermediate product calcium hydrosulfide, which is separated by inclined plate settler to obtain calcium hydrosulfide clear liquid and slag slurry. The slurry reacts with defluorinated wet-process phosphoric acid, and the hydrogen sulfide gas generated is collected through the gas phase outlet. After filtration and separation to remove slag, the clear liquid is reacted with the calcium hydrosulfide aqueous solution obtained from the aforementioned sedimentation separation, and the generated hydrogen sulfide gas is collected through the gas phase outlet, and the pH value of the reaction end point is controlled to be 7.2-9.0. The crude tricalcium phosphate product is obtained by filtration, and then washed and dried to obtain the tricalcium phosphate product. The obtained tricalcium phosphate meets the national...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com