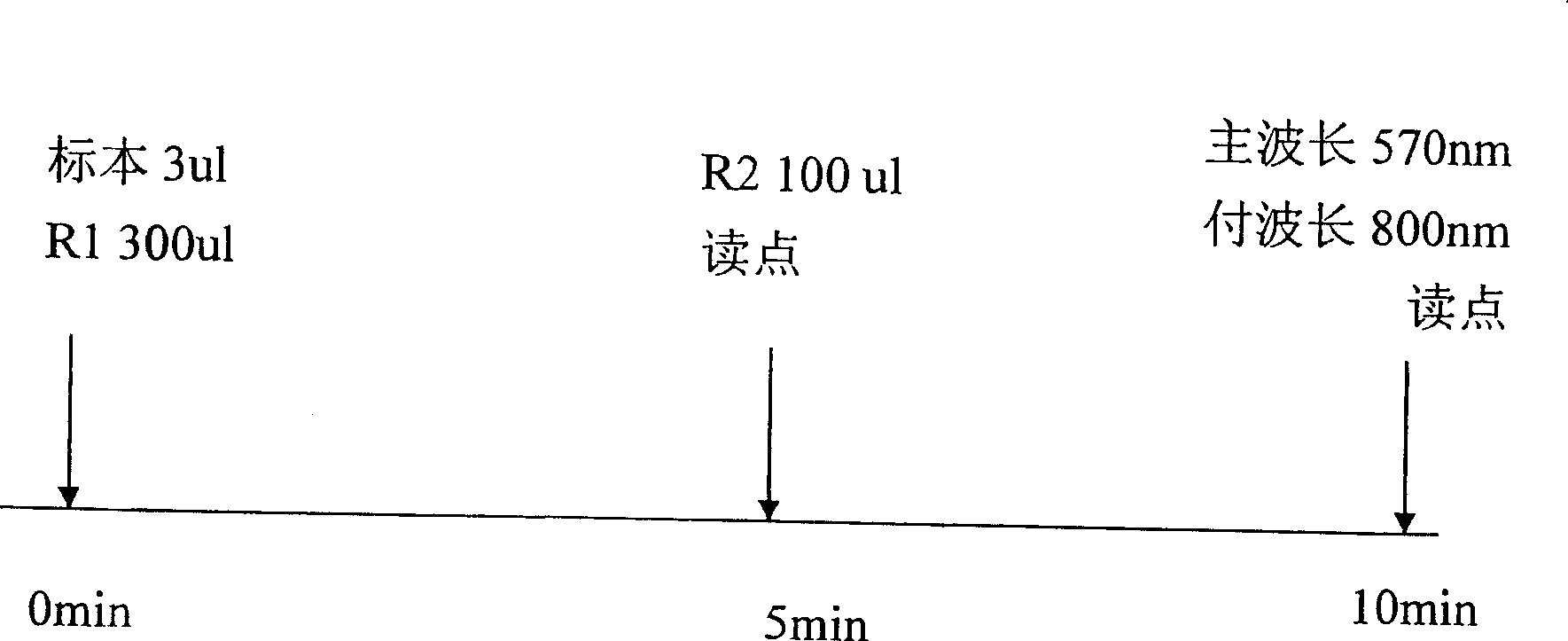
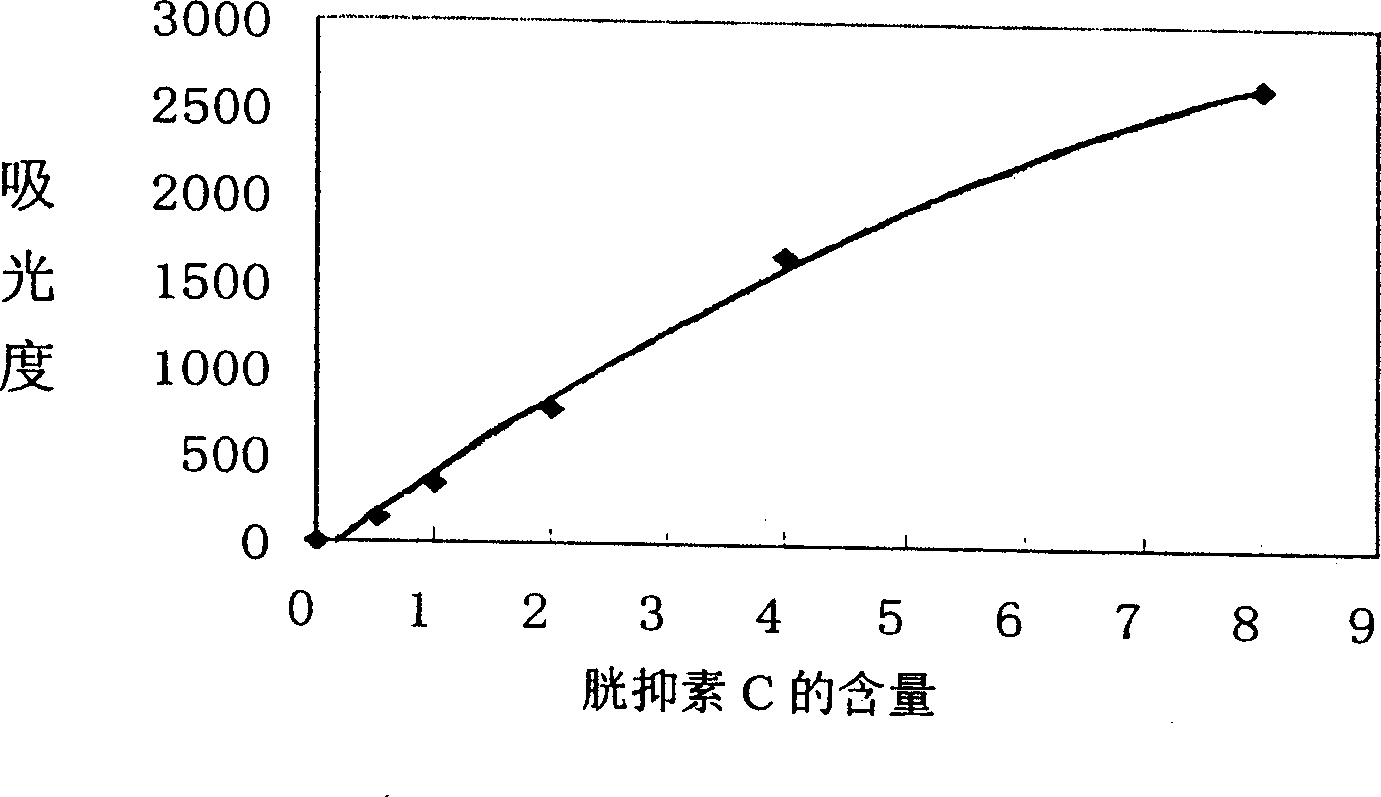
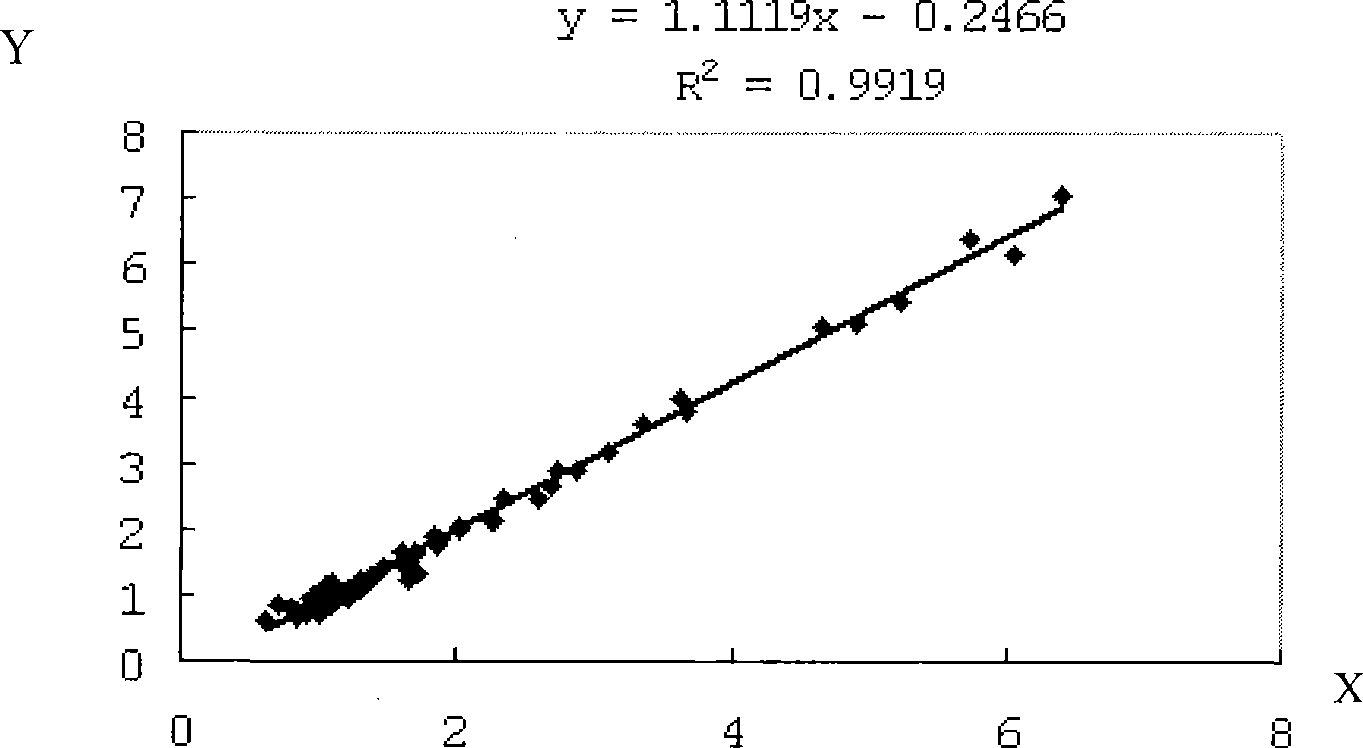
Bladder chalone C determining reagent kit
A technology for detecting kits and cystatin, which is applied in the field of medical immunology in vitro diagnosis, can solve the problems of unavailable measurement, long measurement time, and inability to be fully automated, and achieve high clinical application value, good accuracy, and strong anti-interference ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 detection reagent and standard preparation and assay method
[0021] The main raw materials needed for detection kit of the present invention and standard are as follows:
[0022] 1 Rabbit anti-human cystatin C polyclonal antibody, entrusted to our company to make it according to conventional methods, this antibody only reacts with human cystatin C, has no immune cross-reaction with other antigens, and the titer can meet the requirements of this reagent.
[0023] 2 polystyrene latex, there are many commercial latexes available on the market, including American sigma company and Japanese UNF company; latex particles with various diameters can be selected, and the present embodiment only uses a diameter of 100-150nm as an example Experiments are carried out on latex particles, and the corresponding reagent detection dominant wavelength is 570nm.
[0024] 3 Pure recombinant human cystatin C (purchased from Dako Company) was used to prepare the standard required...
Embodiment 2
[0031] Embodiment 2: Correlation test and sensitivity test of detection reagent
[0032] 1 Correlation test
[0033] Respectively use the reagent of the present invention (the specific formula is the same as in Example 1) and the commercially available cystatin C latex-enhanced reagent of Company A, and adopt the Olympus AU400 automatic biochemical analyzer to test 50 parts of human serum (including normal and abnormal specimens) were measured at the same time according to their respective parameters. The main parameters of our reagents were the same as above, that is, the determination method of cystatin C reagent. Correlation analysis was carried out on the measured values (see results in image 3 , X and Y axes are measured values (mg / L of cystatin C content). The correlation coefficient of the two tests is R 2 =0.9919, the regression equation is y=1.1119x-0.2466. The results show that this reagent has a good correlation with imported reagents in the determination of...
Embodiment 3
[0047] Embodiment 3: the anti-interference test of detection reagent
[0048] The present invention has carried out anti-interference test to detection reagent (specific formula is the same as embodiment 1) and control reagent simultaneously, and test result is as shown in table 3, after adding interfering substance, the relative error to various interfering substances is all within 10%. . The relative errors of the control reagents for various interfering substances are all more than 10%. It shows that even if there is a certain concentration of interfering substances including hemoglobin, bilirubin, Vc, triglyceride, etc. in the detection sample, it has little effect on the measurement results of the present invention.
[0049] table 3:
[0050] interferer This reagent Relative error A company reagent Relative error sample+water 0.48 ----- 0.61 ---- Sample+1600mg / dl glycerol
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com