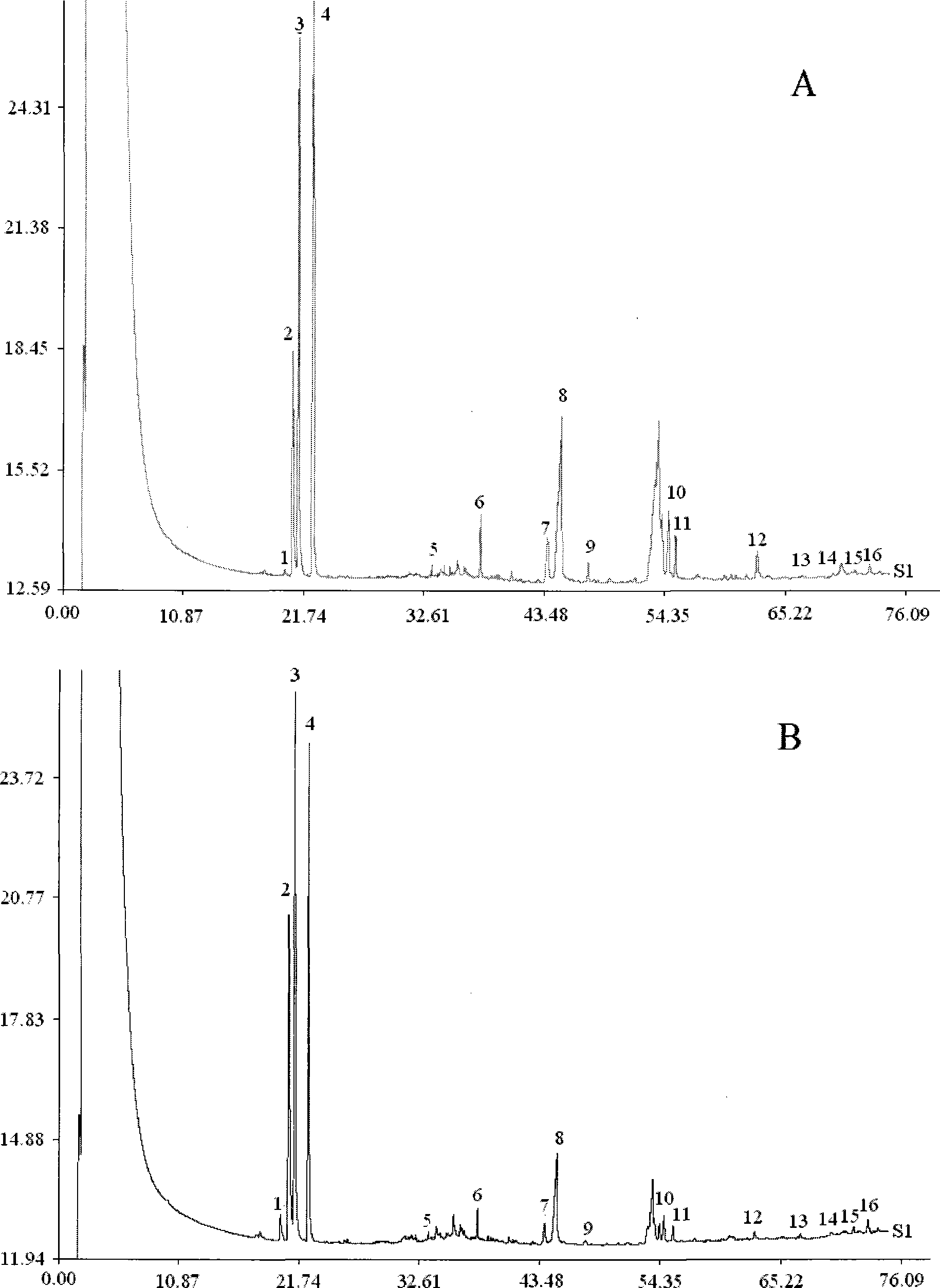Quality control method of volatile ingredients fingerprint of bone knitting medicine of traumatology
A quality control method and technology of volatile components, applied in the field of quality control of gas chromatographic fingerprints of bone medicine preparations in traumatology, can solve problems such as difficult to characterize the physical and chemical characteristics of volatile components, achieve reagent saving, high precision, and reproducibility good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1. Instruments and reagents
[0054] 1.1 Instrument
[0055] Shimadzu 14C gas chromatograph, Zhejiang Zhida N3000 chromatographic workstation, KUDOS ultrasonic cleaner (Shanghai Kedao Ultrasonic Instrument Co., Ltd.).
[0056] 1.2 Reagents and reagents
[0057] Methyl salicylate is sourced from China National Institute for the Control of Pharmaceutical and Biological Products, and Shangke Jiegu Tablets and Shangke Jiegu Capsules are provided by Dalian Merro Traditional Chinese Medicine Factory Co., Ltd. Ethyl acetate and ethanol were of analytical grade
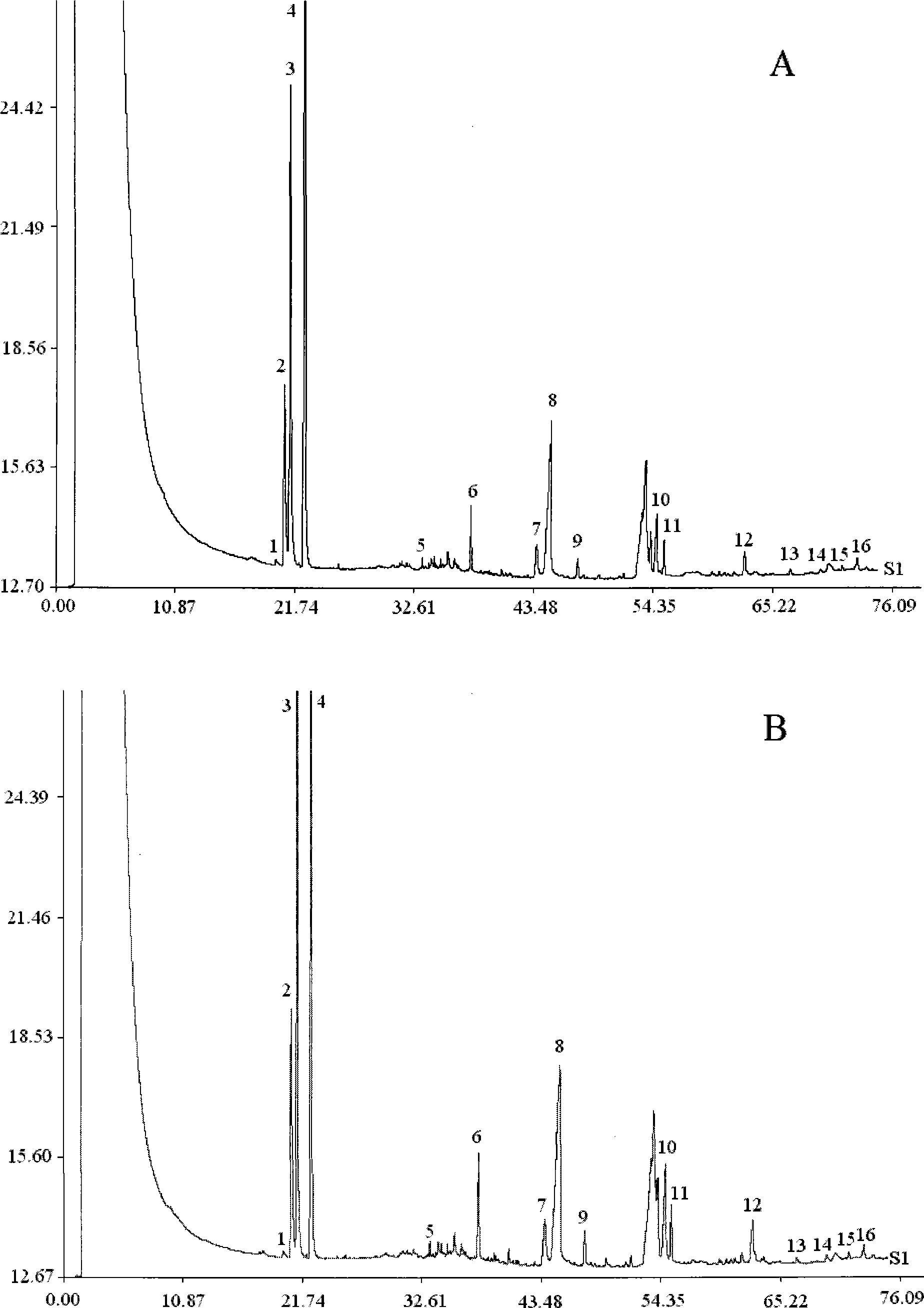
[0058] 2. Determination of standard fingerprints of Shangke Jiegu Tablets and Shangke Jiegu Capsules
[0059] 2.1 Gas chromatography conditions
[0060] DB-5 capillary chromatographic column (30m×0.25mm, 0.25μm); inlet temperature 270°C, detector temperature 290°C; carrier gas is nitrogen, split ratio 20:1, pre-column pressure 120KPa; injection volume 0.5μl . Programmed temperature rise: keep at 60°C for 5 minute...
Embodiment 2
[0077] 1. Determination of standard fingerprints of Shangke Jiegu Tablets and Shangke Jiegu Capsules
[0078] 1.1 Preparation of internal standard solution
[0079] Take an appropriate amount of methyl salicylate, accurately weighed, and use ethyl acetate to make a solution containing 1 mg per 1 ml of the solution as the internal standard solution.
[0080] 1.2 Preparation of the test solution
[0081] Take 2.0g of Shangke Jiegu Tablets or Shangke Jiegu Capsules for comparison, add 6.0ml of ethyl acetate internal standard solution of methyl salicylate, ultrasonically extract for 30min, let cool to room temperature, and shake well to obtain the test solution.
[0082] 1.3 Other conditions are the same as in Example 1
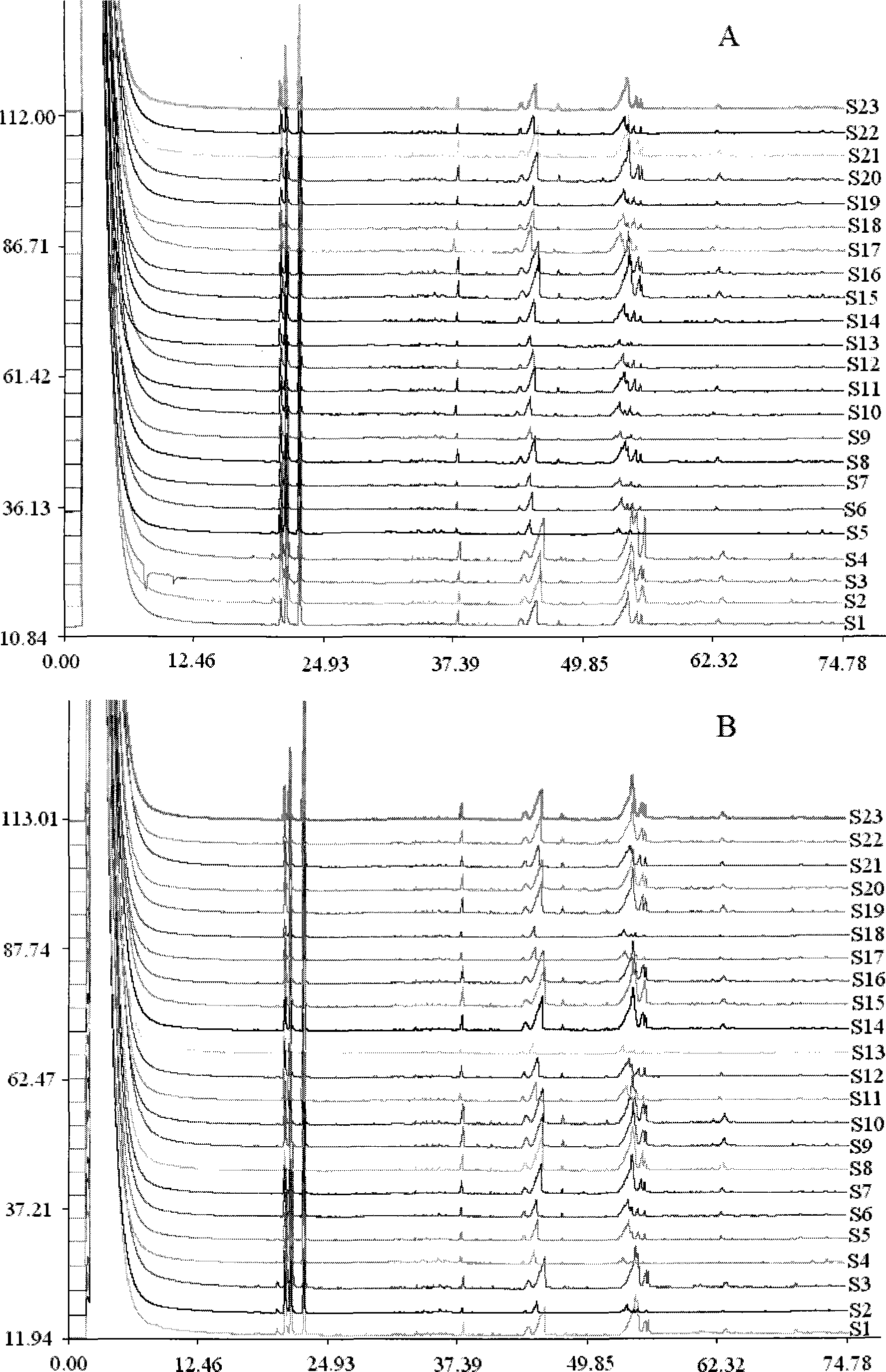
[0083] 2. Determination of fingerprints of Shangke Jiegu Tablets and Shangke Jiegu Capsule samples Select 20 batches of samples of Shangke Jiegu Tablets with different batch numbers and 3 batches of samples of Shangke Jiegu Capsules with different batch numbers...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com