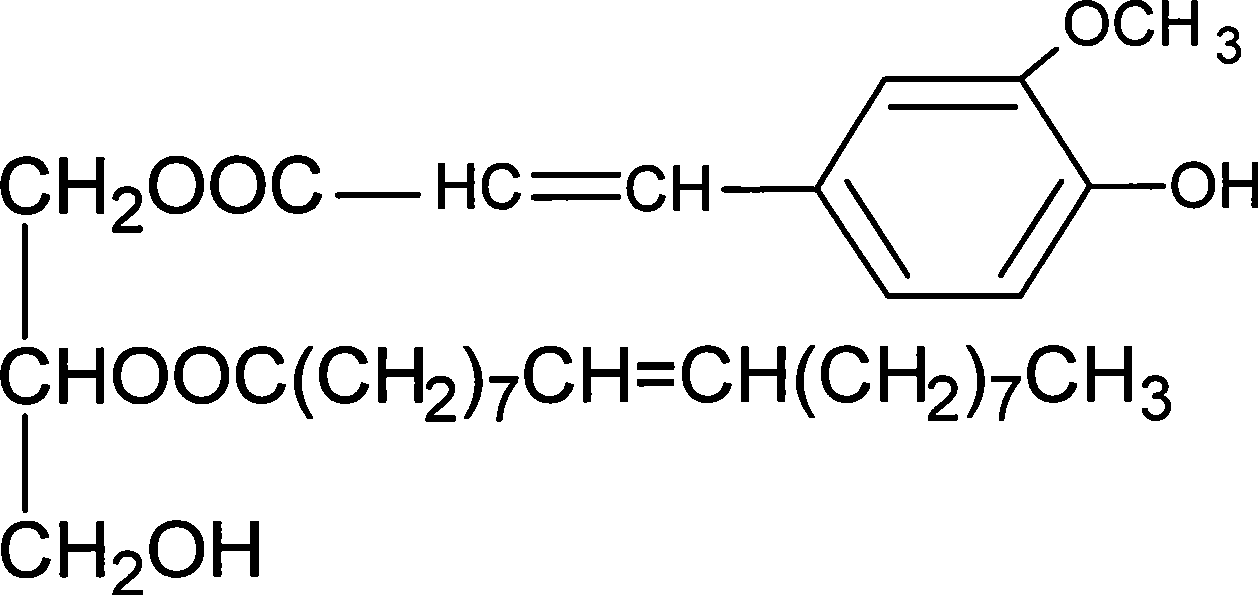
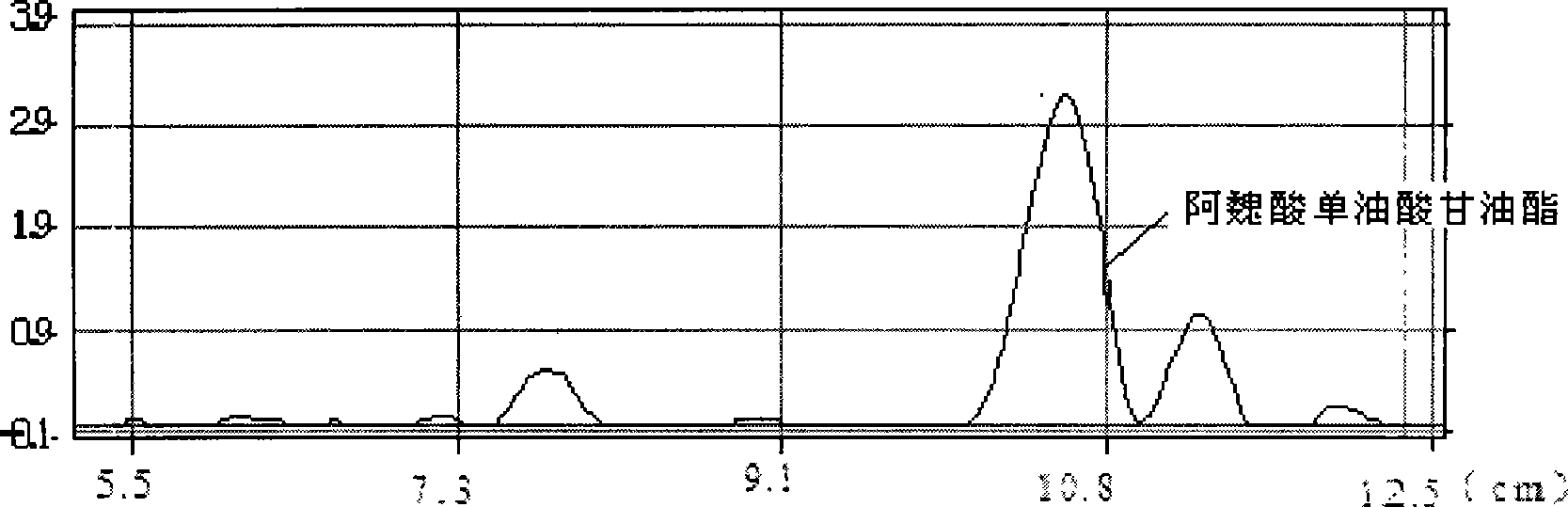
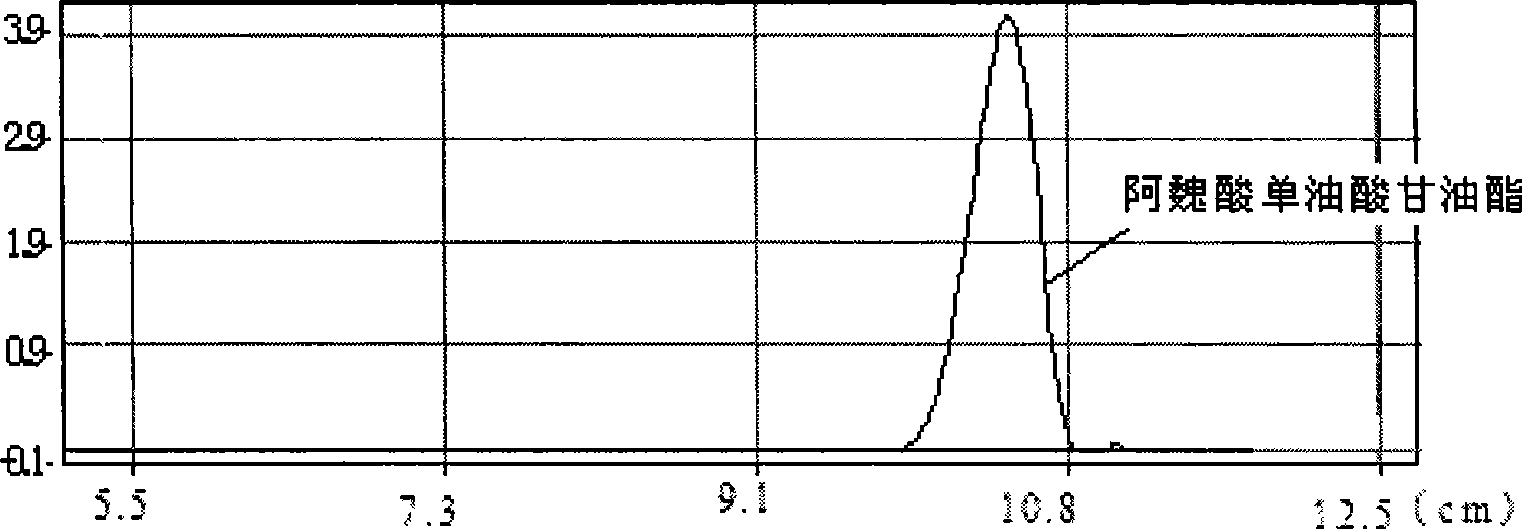
Method for synthesizing ferulaic acid glyceride in surfactant modified organic solvent
A technology of ferulic acid glyceride and surfactant is applied in the field of preparation of ferulic acid glyceride, which can solve the problem that pure ferulic acid glycerol monooleate cannot be obtained, increase the price of ferulic acid monooleic acid glyceride, Downstream technology is complicated and other problems, to achieve the effect of convenient source of raw materials, few steps, and easy purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Dissolve 30g of AOT in 170g of methanol solution, add a certain amount of activated carbon (15g of activated carbon per 100g of AOT), mix and shake for 24 hours, filter and distill under reduced pressure until a small amount of filaments appear, and rectify under reduced pressure to remove methanol , to obtain purified AOT;
[0021] (2) Add 1.11 g of the purified AOT into 50 mL of toluene, shake and react at room temperature for 48 h, and then filter under normal pressure. Take 5mL of the modified toluene solution and add 2mL of isopropanone, if it turns blue, it means AOT modification of toluene is completed;
[0022] (3) Enzyme-catalyzed reaction In a 25 mL Erlenmeyer flask with a stopper, 1 mmoL of ethyl ferulate, 3 mmoL of triolein, 120 mg of Candida rugosa lipase, and 3 mL of AOT-modified toluene were added. The reaction bottle was reacted at 50° C. and 180 rpm in an air bath shaker for 12 hours, and the whole system was carried out under normal pressure durin...
Embodiment 2
[0024] (1) Dissolve 45g of AOT in 255g of methanol solution, add a certain amount of activated carbon (15g of activated carbon per 100g of AOT), mix and shake for 24 hours, filter and distill under reduced pressure until a small amount of filaments appear, and rectify under reduced pressure to remove methanol , to obtain purified AOT;
[0025] (2) Add 1.11 g of the purified AOT to 50 mL of toluene, shake and react at room temperature for 48 hours, and then filter under normal pressure. Take 5mL of the modified toluene solution and add 2mL of isopropanone, if it turns blue, it means AOT modification of toluene is completed;
[0026] (3) Enzyme-catalyzed catalytic reaction In a 25 mL Erlenmeyer flask with a stopper, 2 mmoL of ethyl ferulate, 6 mmoL of triolein, 240 mg of Candida rugosa lipase, and 3 mL of AOT-modified toluene were added. The reaction bottle was reacted at 48° C. and 180 rpm in an air bath shaker for 12 hours, and the whole system was carried out under normal pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com