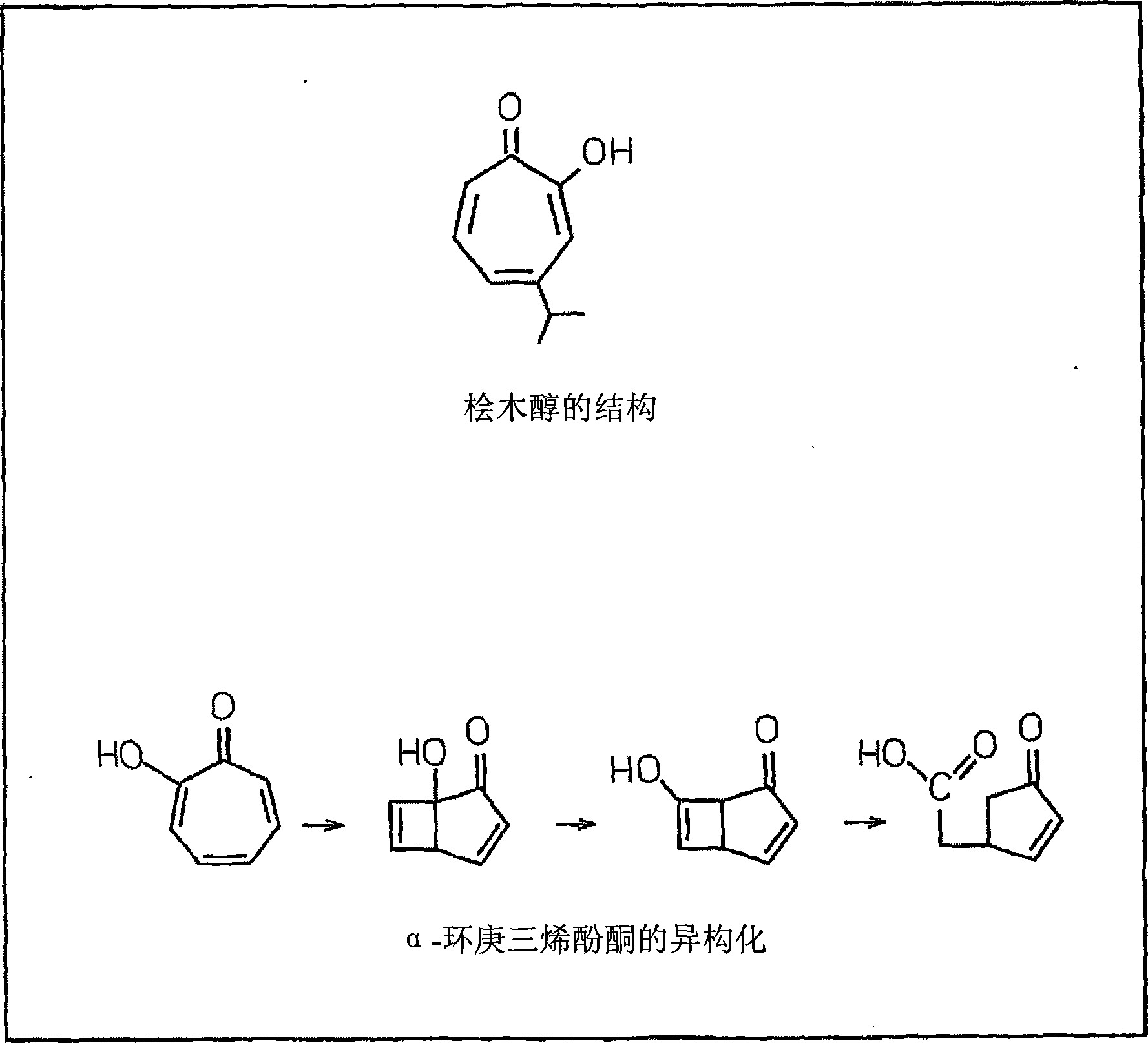
Stabilizer for hydrophobic compounds
A technology of hydrophobic compounds and stabilizers, applied in the directions of active ingredients of hydroxyl compounds, non-active ingredients of polymer compounds, drug combinations, etc., can solve problems such as insufficient stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Stabilization of hinokitiol under the action of block copolymer
[0058] Weigh 1 mg of hinokitiol (Wako, Osaka) and 10 mg of PEG-PBLA-5-20-100, PEG-PBLA-12-40-50, or PEG-PBLA-12-50-50 in a screw cap test tube bottle , after dissolving in dichloromethane, dichloromethane was volatilized under a nitrogen stream to obtain a film formed of hinokitiol and block copolymer. 3 ml of ultrapure water was added to the film, and stirred at 4° C. for 12 hours under light-shielding. The obtained microcapsule solution was irradiated with ultrasonic waves for 5 minutes, the particle diameter was measured, and the hinokinaki alcohol not encapsulated in the microcapsules was separated by gel filtration (PD-10 columns). Hinokitiol has a maximum absorption peak at 330nm in water, and hinokitiol wrapped in block copolymer microcapsules has maximum absorption peaks at 324nm and 372nm. The following table 1 shows the embedding rate (the ratio of the amount of drug embedded in the...
Embodiment 2
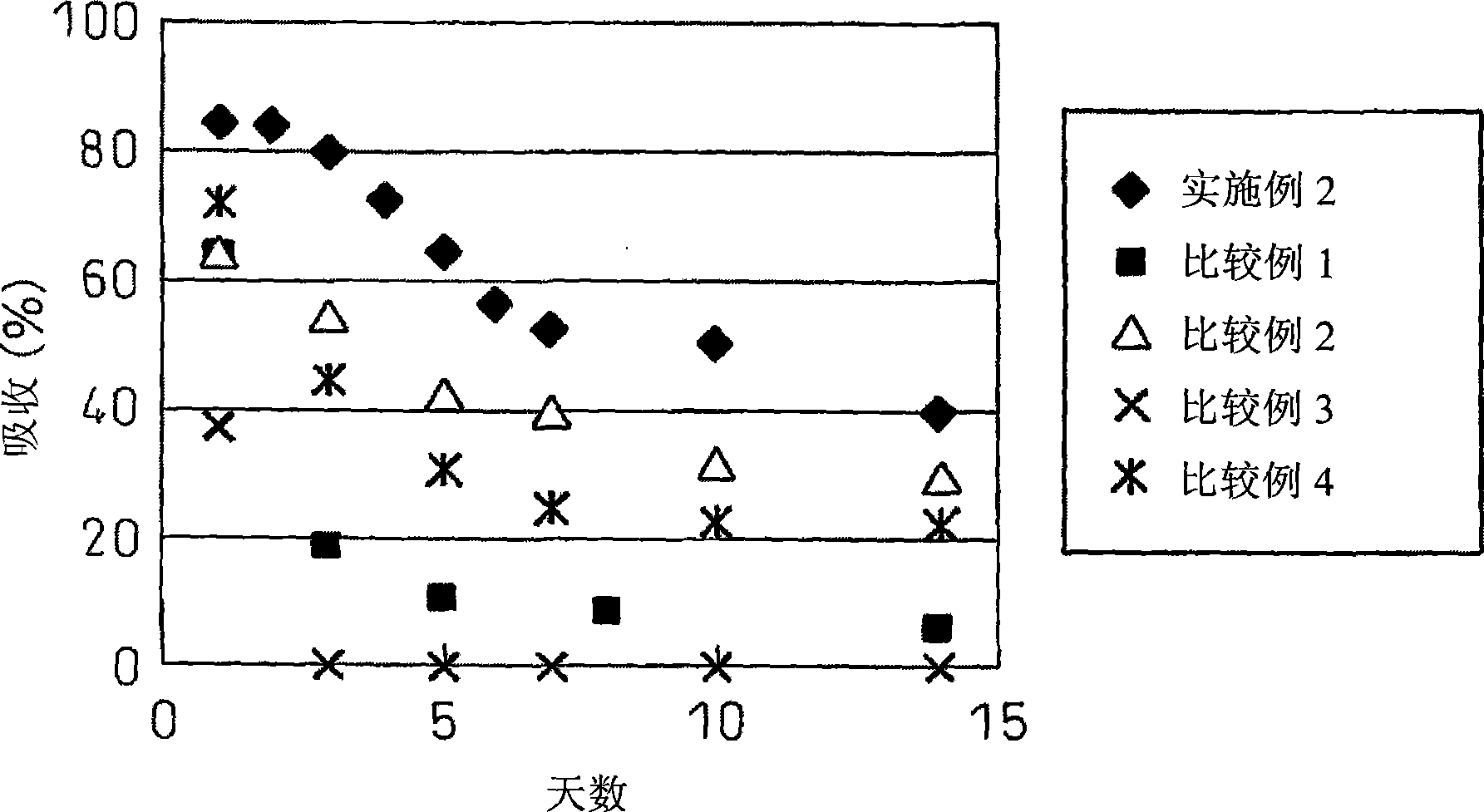
[0064] Example 2 The stability of Hinokitiol under the action of block copolymer to light
[0065] The aqueous solution of microencapsulated hinokitiol (the sample of experiment 1-6 shown in table 1), under the light of laboratory, preserves at room temperature, to their absorption intensity change through time measurement, as figure 2 As shown, the absorption intensity at 324 nm was maintained at 85% after one day, 53% after 7 days, and 40% after 14 days, indicating that the photolysis of hinokinol was inhibited by microencapsulation. again, figure 2 The results shown represent the average of the absorbance at 324 nm for the microcapsules of experiments 1-6. That is, it can be considered figure 1 The isomerization reaction shown is sterically hindered by microencapsulation. In addition, the hindrance of this isomerization reaction was also observed when hinokitiol / block polymer was 1mg / 1.2mg as the starting substance (that is, hinokitiol was in a state of excess relative...
Embodiment 3
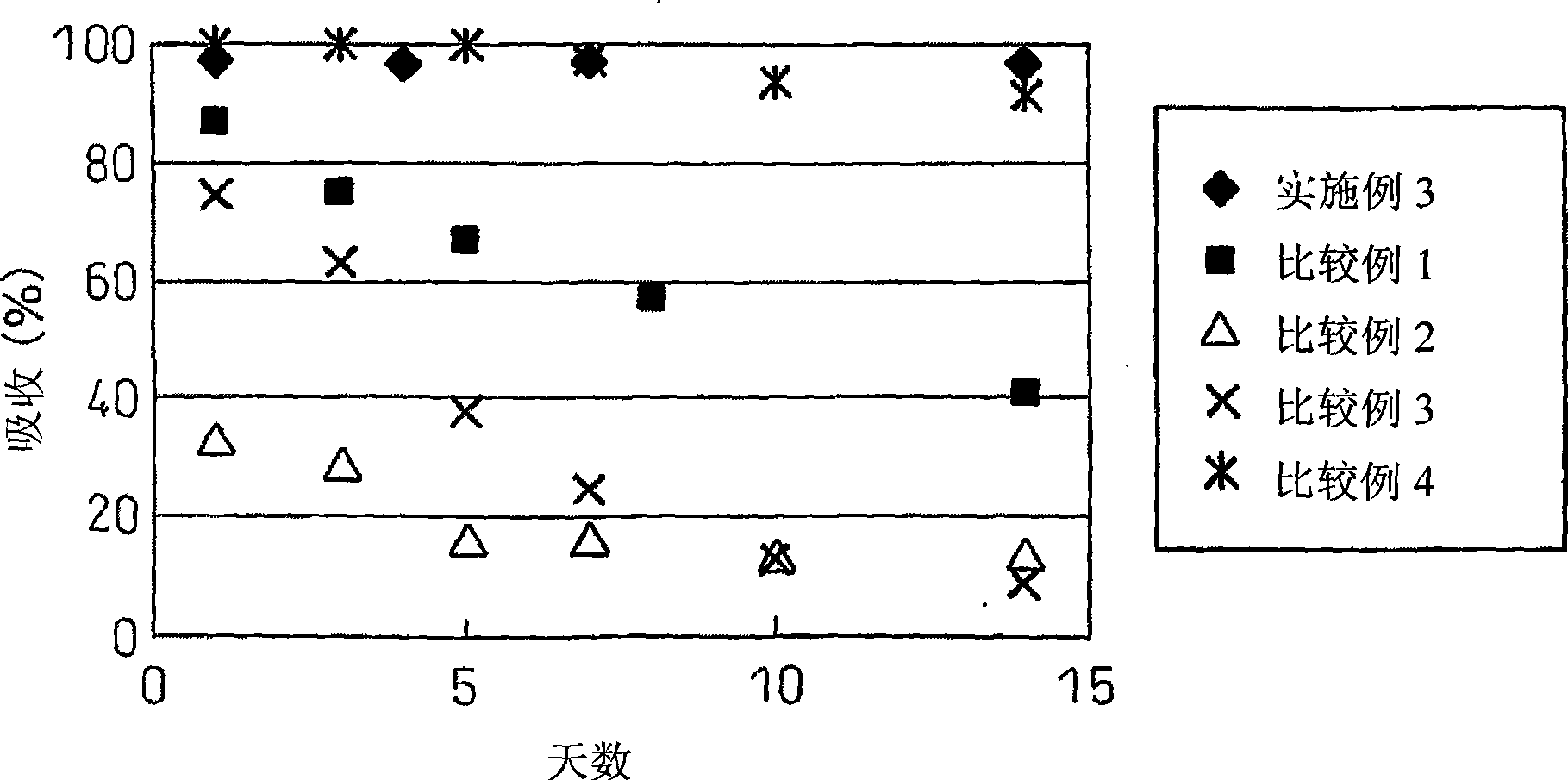
[0066] Example 3: Thermal Stability of Microencapsulated Hinokitiol under the Effect of Block Copolymer
[0067] The aqueous solution of microencapsulated hinokitiol (the sample of experiment 1-6 shown in table 1) is stored under shading at 37°C, and their absorption intensity changes are measured over time, and the absorption maximum at 324nm is after 14 days Still remain at 97%, show that microencapsulation effect makes the thermal decomposition of hinokitiol be suppressed completely ( image 3 ). Since the spectrum in the case of thermal decomposition is exactly the same as the spectrum in the case of photolysis, it can be considered that the decomposition products in the case of photolysis and thermal decomposition are cyclopentene derivatives, and that the inhibition of thermal decomposition and the inhibition of photolysis are caused by the same caused by the mechanism.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com