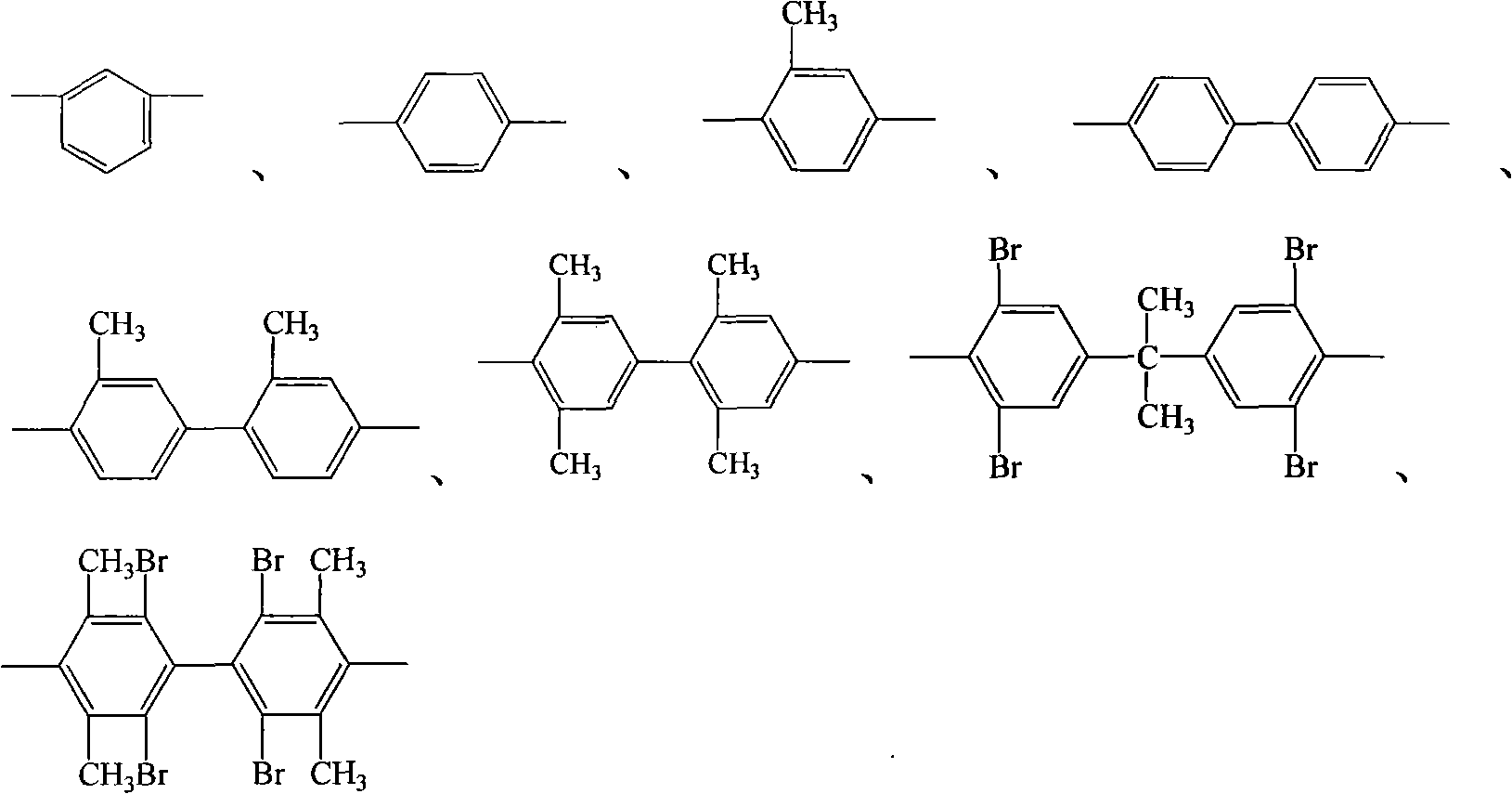
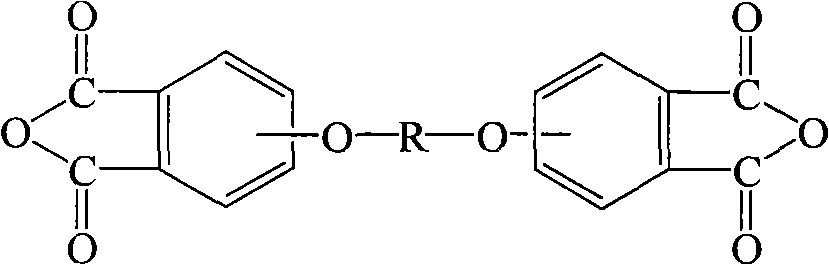Process for producing aromatic diaether dianhydride monomer
A dianhydride monomer and aromatic technology, used in the synthesis of dianhydride monomers, can solve problems such as high sealing requirements, easy oxidation of phenol, and reduced conversion rate of phthalic anhydride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Add 11.2 g KOH and 160 g absolute ethanol into a 500 ml three-neck flask, stir until dissolved; then add 38.4 g 4-nitrophthalimide, stir at 25°C for 4 hours; filter after the reaction, collect the mother liquor, and distill under reduced pressure Recover absolute ethanol; vacuum-dry the filter cake (potassium salt) at 80°C.
[0087] Add the potassium salt prepared above into a 500ml three-neck flask, then add 25.3g benzyl chloride and 100gDMF, raise the temperature to 130°C, and react at a constant temperature for 4h; Purify and dry under vacuum at 120°C for 2 hours to obtain 4-nitro-N-benzylphthalimide.
[0088]Alternatively, add 51.8gK to a 500ml three-necked flask 2 CO 3 , 57.9g of 4-nitrophthalimide, 75.9g of benzyl chloride and 200g of DMF, stirred and heated up to 140°C for 6 hours at a constant temperature; after the reaction was completed, the temperature was lowered to 50°C, solids were precipitated, filtered, and firstly used 2% Wash with dilute hydrochlori...
Embodiment 2
[0093] Add 10.5g KOH and 130g absolute ethanol into a 500ml three-neck flask, stir until dissolved; then add 36.0g 3-nitrophthalimide, stir at 35°C for 4 hours; filter after the reaction, collect the mother liquor, and distill under reduced pressure Recover absolute ethanol; vacuum-dry the filter cake (potassium salt) at 85°C.
[0094] Add the potassium salt prepared above into a 500ml three-neck flask, then add 23.7g of benzyl chloride and 90g of DMF, raise the temperature to 140°C, and react at a constant temperature for 6h; Purify and dry in vacuum at 120°C for 2 hours to obtain 3-nitro-N-benzylphthalimide.
[0095] Add 19.1g of biphenol into a 500ml three-necked flask, blow nitrogen, add 125ml of dimethyl sulfoxide, 18.7g of 50% NaOH aqueous solution, then add 75ml of toluene, reflux to remove water for 5 hours, and distill the toluene out after the water separation is completed . Cool to 55°C, add 29.3g of the above-prepared product 3-nitro-N-benzylphthalimide, 1.5g tet...
Embodiment 3
[0099] Add 13.5g KOH and 140g absolute ethanol into a 500ml three-neck flask, stir until dissolved; then add 46.3g 4-nitrophthalimide, stir at 30°C for 5h; filter after the reaction, collect the mother liquor, and distill under reduced pressure Recover absolute ethanol; filter cake (potassium salt) is vacuum-dried at 90°C.
[0100] Add the potassium salt prepared above into a 500ml three-neck flask, then add 32.8g of bromobutane and 90g of DMF, raise the temperature to 140°C, and react at a constant temperature for 6h; Purified with ethylene glycol methyl ether and dried in vacuum at 100°C for 2 hours to obtain 4-nitro-N-butylphthalimide.
[0101] Add 22.8g bisphenol A into a 500ml three-neck flask, blow nitrogen, add 100ml dimethyl sulfoxide, 16g 50% NaOH aqueous solution, then add 50ml toluene, reflux to remove water for 6 hours, and distill the toluene out after the water separation is completed. Cool to 70°C and add 45.3g of the product 4-nitro-N-butylphthalimide prepared...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com