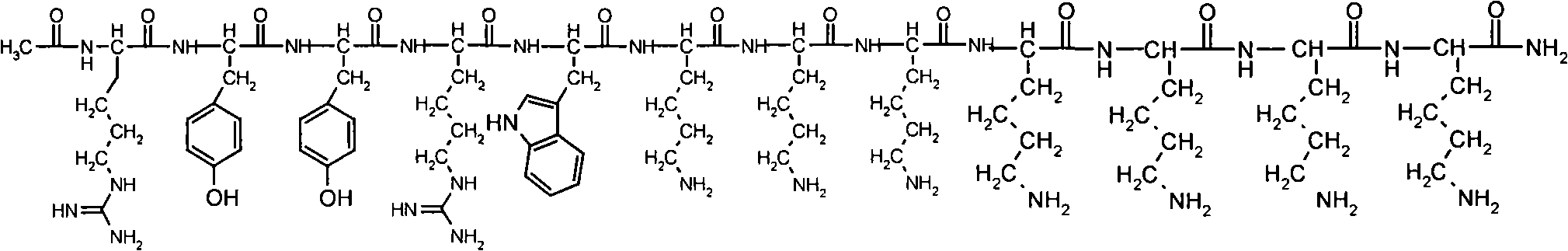
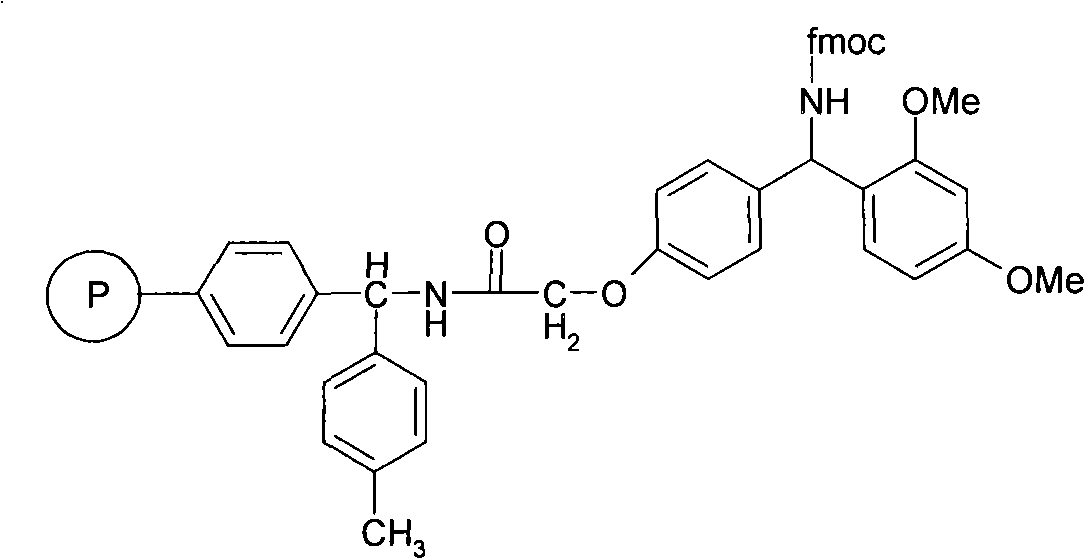
Solid phase synthesis method of ZP120
A technology of ZP120 and solid-phase synthesis, which is applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of long production cycle, lengthy route, high cost, etc., and achieve industrial production, good product quality, and low spin effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: Preparation of Fmoc-Lys(Boc)-Rink Amide MBHA
[0053] operate:
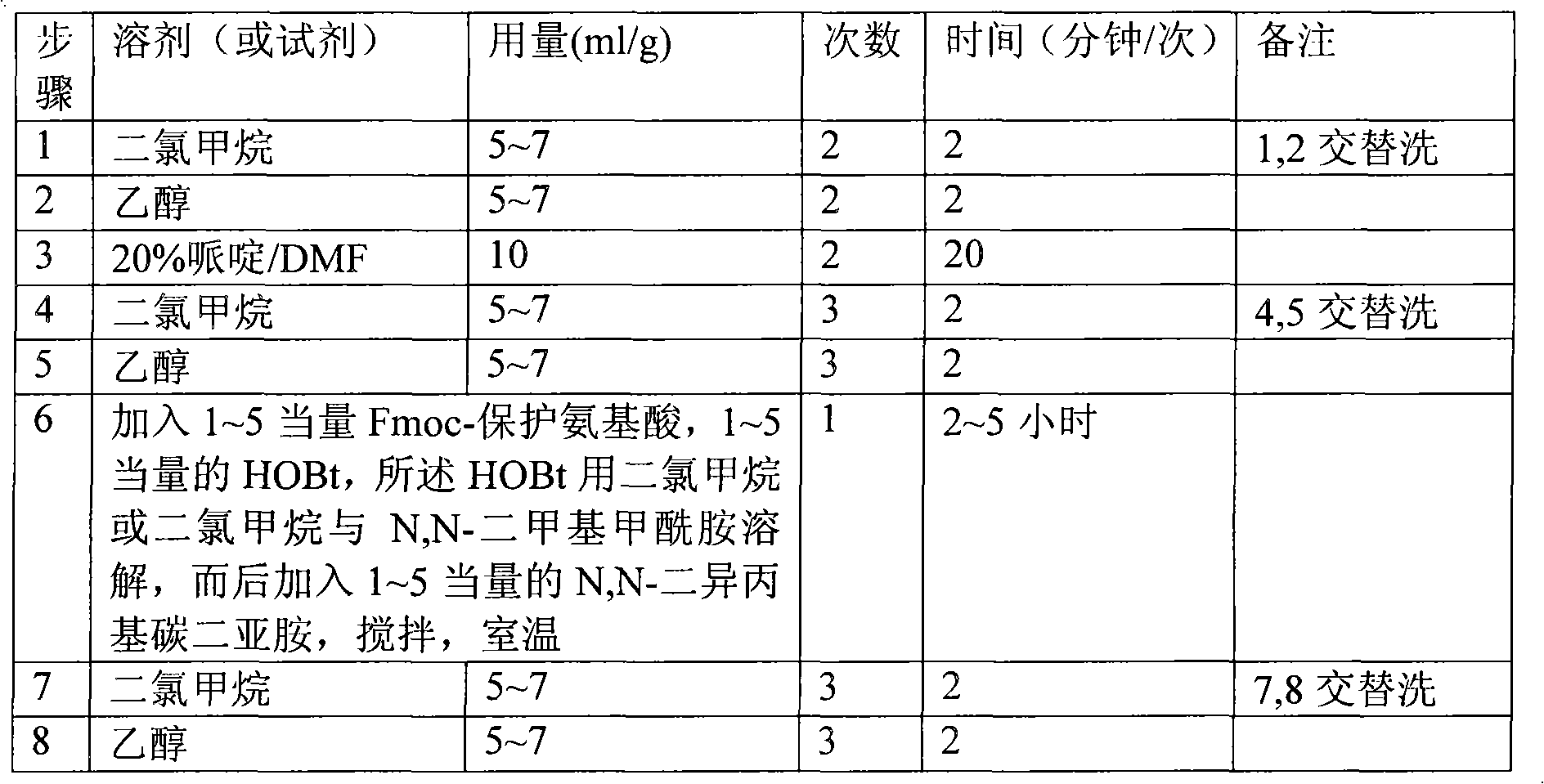
[0054] (1) Put 50g (30mmol) of Fmoc-Rink Amide-MBHA resin in the polypeptide synthesis reactor, alternately wash twice with 300ml of dichloromethane and 300ml of ethanol, 2 minutes each time, and drain. Add 500 ml of 20% (v / v) piperidine / DMF solution, stir and react at room temperature for 20 minutes, and drain. The resin was alternately washed 3 times with dichloromethane and 300ml ethanol, 2 minutes each time, and drained. Kaiser reagent test, the result was positive, Fmoc- has been removed.
[0055] (2) After dissolving 70.28g of Fmoc-Lys(Boc)-OH and 20.25g of HOBt with 500ml of dichloromethane / DMF solution (the volume ratio of dichloromethane and DMF is 1:1, the same below), add them to the resin, and then Add 23 ml of N,N-diisopropylcarbodiimide dropwise, and stir at room temperature for 5 hours after the drop is complete.
[0056] (3) Drain. The resin was alternately washed 3 times...
Embodiment 2
[0059] Embodiment 2: Preparation of Fmoc-Lys(Boc)-Lys(Boc)-Rink Amide MBHA
[0060] operate:
[0061] (1) Add 500 ml of 20% piperidine / DMF solution to the resin, stir and react at room temperature for 20 minutes, and drain. The resin was alternately washed 3 times with dichloromethane and 300ml ethanol, 2 minutes each time, and drained. Kaiser reagent test, the result was positive, Fmoc- has been removed.
[0062] (2) After dissolving 70.28g of Fmoc-Lys(Boc)-OH and 20.25g of HOBt with 500ml of dichloromethane / DMF solution, add it to the resin, then add 23ml of N,N-diisopropylcarbodiimide dropwise, After the drop was completed, it was stirred at room temperature for 5 hours.
[0063] (3) Drain. The resin was alternately washed 3 times with 300ml of dichloromethane and 300ml of ethanol, each time for 2 minutes, and drained.
[0064] (4) Kaiser reagent was used for color development, and the test was negative. The reaction is over.
[0065] Get: Fmoc-Lys(Boc)-Lys(Boc)-Rink...
Embodiment 3
[0066] Embodiment 3: Preparation of Fmoc-Lys(Boc)-Lys(Boc)-Lys(Boc)-Rink Amide MBHA
[0067] operate:
[0068] (1) Add 550 ml of 20% piperidine / DMF solution to the resin, stir and react at room temperature for 20 minutes, and drain. The resin was alternately washed 3 times with dichloromethane and 350 ml of ethanol, 2 minutes each time, and drained. Kaiser reagent test, the result was positive, Fmoc- has been removed.
[0069] (2) After dissolving 70.28g of Fmoc-Lys(Boc)-OH and 20.25g of HOBt with 500ml of dichloromethane / DMF solution, add it to the resin, then add 23ml of N,N-diisopropylcarbodiimide dropwise, After the drop was completed, it was stirred at room temperature for 5 hours.
[0070] (3) Drain. The resin was alternately washed 3 times with 350ml of dichloromethane and 350ml of ethanol, each time for 2 minutes, and drained.
[0071] (4) Kaiser reagent was used for color development, and the test was negative. The reaction is over.
[0072] Get: Fmoc-Lys(Boc)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com