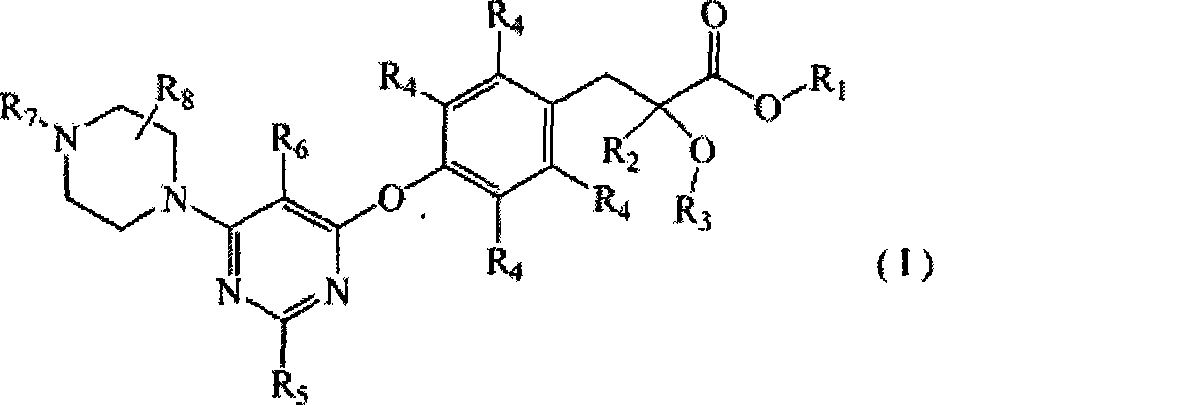
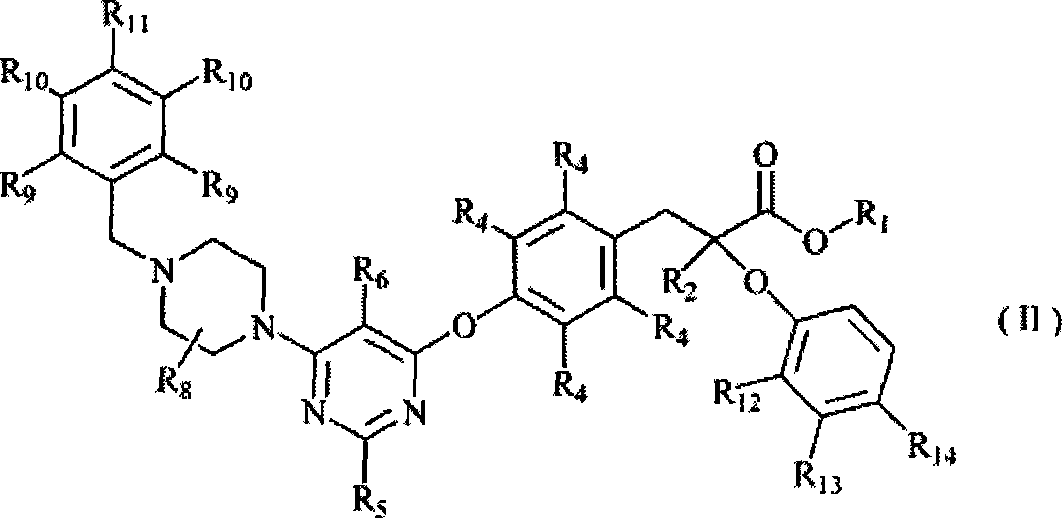
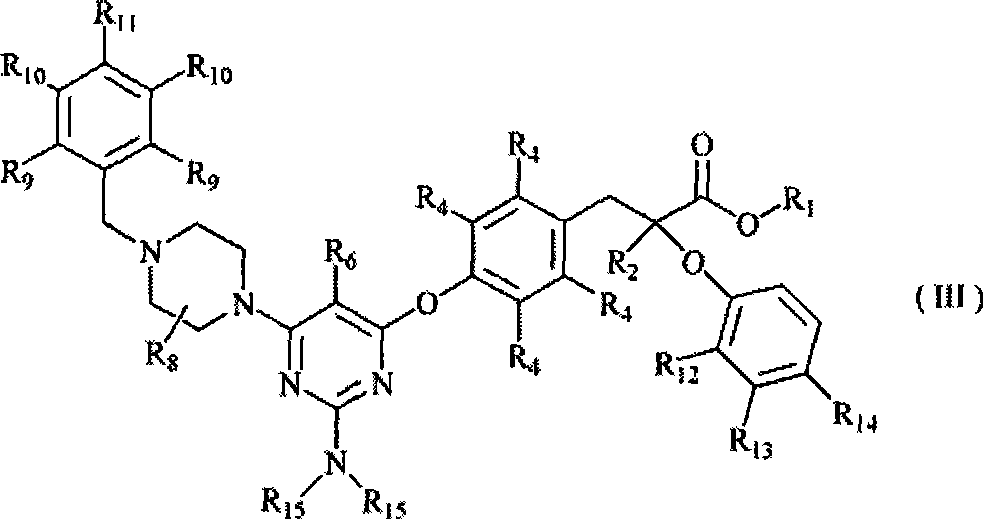
Use of pyrimidine-substituted phenylpropionic acid compound in preparing medicament for preventing and/or treating diabetes
A compound, the technology of phenylpropionic acid, which is applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problems of phenylpropionic acid compounds without pyrimidine substitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 3-{4-[2-Amino-6-(4-benzyl-piperazin-1-yl)-pyrimidinyl-4-oxo]-phenyl}-2-ethane Oxy-phenylpropionic acid (Compound 1)
[0072]
[0073] P(OEt) 2 (2-Ethoxy-2-(diethoxyphosphoryl)-ethyl acetate) was added to a mixed solution of methyl tert-butyl ether (55mL) and tert-butyl potassium (4.65g) under nitrogen protection , p-benzyloxybenzaldehyde (4.61g) was added to the mixture at 5°C, then tert-butanol (6.70g) was added, and the reaction was carried out at 15°C for about 30min. TLC detected that the reaction was complete, and water (30mL ), rotary evaporated the organic phase, added ethanol (30mL), recrystallized, washed with ethanol / water (1:1v / v), and dried to obtain 2-ethoxy-1-(4-benzyl)phenylacrylic acid ethyl Esters, 92% yield.
[0074] 1 HNMR, 400MHz (acetone-d6): δ1.12(t, J=7Hz), 1.32(t, J=7Hz), 1.33(t, J=7Hz), 3.91(q, J=7Hz), 4.12(q , J=7Hz), 4.24(q, J=7Hz), 5.12(s), 5.17(s), 6.10(s), 6.93(s), 6.94(d, J=9Hz), 7.05(d, J= 9Hz), 7.15(d, J=9Hz), 7.32~7...
Embodiment 2
[0079] Example 2 3-{4-[2-Amino-6-(4-benzyl-piperazin-1-yl)-pyrimidinyl-4-oxo]-phenyl}-2-methyl yl-2-phenoxypropionic acid (compound 2)
[0080]
[0081] Dissolve ethyl 2-phenoxypropionate (15g) in anhydrous THF, add lithium diisopropylamide (26ml) at -78°C, stir for half an hour, add p-benzyloxybenzaldehyde ( 10 g), stirred overnight under nitrogen protection. Add NH at room temperature 4 Cl saturated liquid until there are no bubbles, the aqueous layer is extracted twice with ethyl acetate, the organic phases are combined, rotary evaporated, passed through the column, PE:EA=10:1 to 4:1, and a light yellow solid 3-(4-benzyl Oxy)phenyl-3-hydroxy-2-methyl-2-phenoxypropanoic acid ethyl ester, 50% yield. Under ice-cooling, add 3-(4-benzyloxy)phenyl-3-hydroxy-2-methyl-2-phenoxy ethyl propionate (10g) and dichloromethane into the three-necked flask, Slowly drive the barrel into the BF 3 -Et 2 O(3.06ml) and (C 2 h 5 ) 3 SiH (4.08ml), into a blood-red liquid, overnight,...
Embodiment 3
[0088] Example 3 S-3-{4-[2-amino-6-(4-benzyl-piperazin-1-yl)-pyrimidinyl-4-oxo]-phenyl}-2- Methyl-2-phenoxypropionic acid (Compound 3)
[0089]
[0090] 3-{4-[2-Amino-6-(4-benzyl-piperazin-1-yl)-pyrimidinyl-4-oxo]-phenyl}-2-methyl-2-phenoxy Ethyl propionate was resolved by chiral preparative column to obtain S-3-{4-[2-amino-6-(4-benzyl-piperazin-1-yl)-pyrimidinyl-4-oxo]- Ethyl phenyl}-2-methyl-2-phenoxypropionate. ee: 99% (AD-H: 0.46 cm I.D. x 25 cm).
[0091]S-3-{4-[2-Amino-6-(4-benzyl-piperazin-1-yl)-pyrimidinyl-4-oxo]-phenyl}-2-methyl Dissolve ethyl-2-phenoxypropionate (1 mmol), add KOH supersaturated solution, overnight, rotary evaporate THF and methanol, add HCl to adjust pH = 6, a white solid precipitates, filter, wash with a small amount of water, use the water layer THF was extracted twice, the organic layers were combined and rotary evaporated to obtain a white solid, which was recrystallized from methanol to obtain S-3-{4-[2-amino-6-(4-benzyl-piperazin-1-yl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com