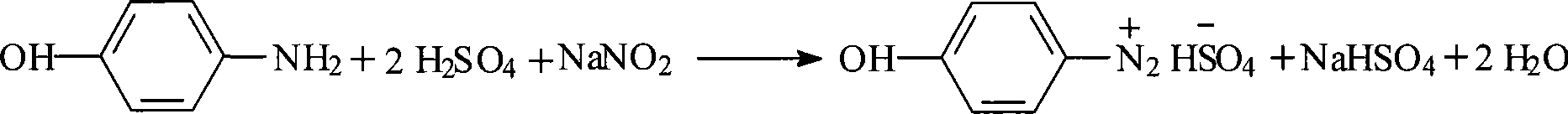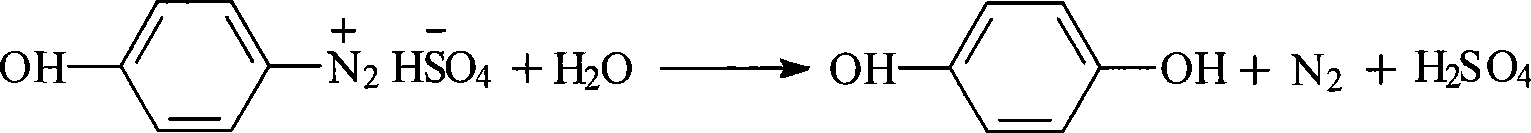Method for preparing p-dihydroxy benzene
A technology of hydroquinone and p-aminophenol, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as equipment corrosion, difficulty in selecting equipment materials, harsh conditions, etc., and achieve the goal of increasing production capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Hydroquinone preparation method carries out according to the following steps:
[0045] Diazotization reaction: Add 408Kg (0.625Kmol) of 15% sulfuric acid and 27.8Kg (0.25Kmol) of industrial product 98% p-aminophenol into a 1000L reactor, stir and cool down to -5~0°C, add 40% sodium nitrite dropwise 48Kg (0.275Kmol) aqueous solution, adding time for 30-45min, keep warm for 0.5hr after dropping, add 1.5Kg urea, stir for 15min, when the release of nitrogen is not obvious, add 1.5Kg thiourea and stir for another 15min. The dropwise addition, heat preservation and feeding temperature are all kept at -5-5°C to obtain 470-480Kg of dark brown diazonium liquid, which is stored at 0°C.
[0046] Hydrolysis reaction: Add 1000Kg of 30% sulfuric acid to a 2000L reactor, heat to reflux at 106-108°C, add the above diazo solution dropwise at about 0°C for 1-2 hours, then reflux at 102-104°C for 3- 4hr, the temperature was lowered to obtain 1460Kg of light brown hydrolyzate, and the hyd...
Embodiment 2 to Embodiment 7
[0052] The preparation method is the same as in Example 1, except that the concentration of sulfuric acid in the diazotization reaction is different.
[0053]
[0054]
[0055] Increase the diazotization acidity from 10% to more than 30%, the color of the diazonium solution and the hydrolyzed solution becomes darker and darker, and the diazonium salt is easy to precipitate at low temperature, causing unsafe factors.
Embodiment 8 to Embodiment 12
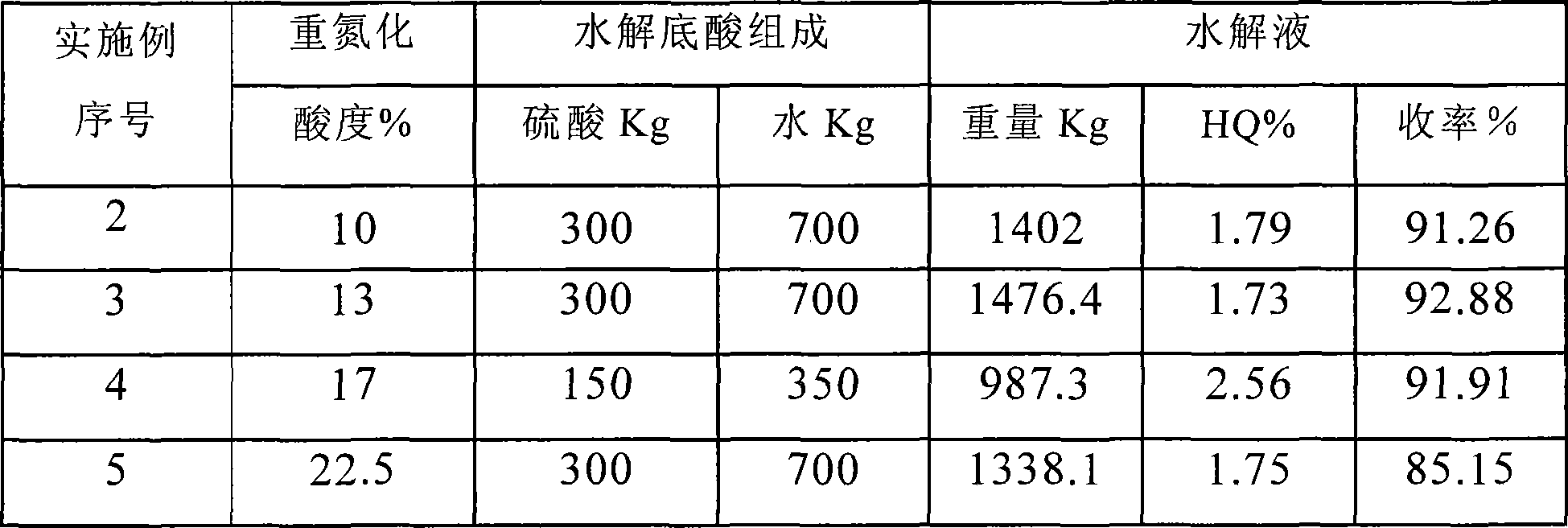
[0057] The preparation method is the same as in Example 1, except that the ratio of hydrolyzed base acid is changed. The composition of sulfuric acid / water (kg / kg) in the bottom acid is ①300 / 700; ②300 / 450; ③150 / 350; ④300 / 450; ⑤300 / 300.
[0058]
[0059] When the hydrolyzed base acid concentration is 30-40%, the change of the base acid composition has little effect on the yield, and the oil layer yield is above 90%; when the base acid concentration is increased to 50%, the yield drops obviously.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com