Culture medium for composite enrichment of salmonella, Listeria monocytogenes and Staphylococcus aureus, and preparation thereof
A technology for Listeria monocytogenes and Salmonella, which is applied in the direction of biochemical equipment and methods, measurement/testing of bacteria and microorganisms, etc., can solve the problems of unsuitable high background microbial raw materials, lack of inhibitors, etc., and shorten the enrichment time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
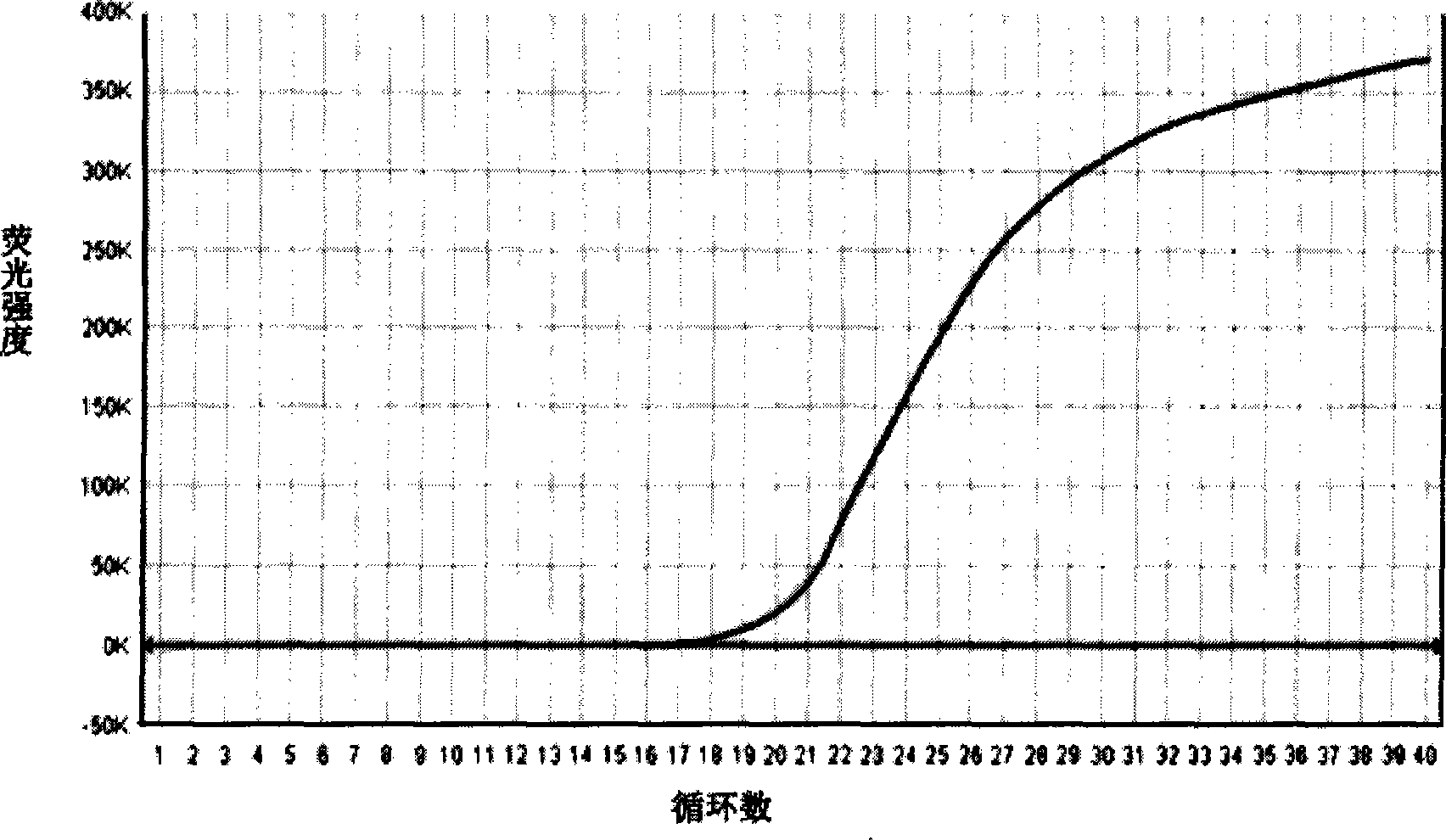
[0020] Weigh tryptone 17g, peptone 3g, sodium dihydrogen phosphate 2.5g, glucose 2.5g, escin 1g, sodium pyruvate 2.5g, mannitol 5g, sodium chloride 20g, lithium chloride 2g, distilled water and add to 990ml, Heat to dissolve, cool to room temperature and correct the pH to 7.2-7.3; mix well, sterilize at 121°C for 15 minutes; weigh 10 mg of nalidixic acid and add it to 5 ml of sodium hydroxide solution with a concentration of 0.05 mol / L to obtain naphthyridine Keto acid solution. Weigh 0.15 mg of potassium tellurite into 5 ml of sterilized distilled water to prepare a potassium tellurite solution. Under aseptic conditions, add 5 ml of nalidixic acid solution and 5 ml of potassium tellurite solution; subpackage and store at 4°C for later use, to prepare a compound enrichment for Salmonella, Listeria monocytogenes and Staphylococcus aureus. culture medium.
[0021] Dilute the prepared medium for Salmonella, Listeria monocytogenes and Staphylococcus aureus compound enrichment to...
Embodiment 2
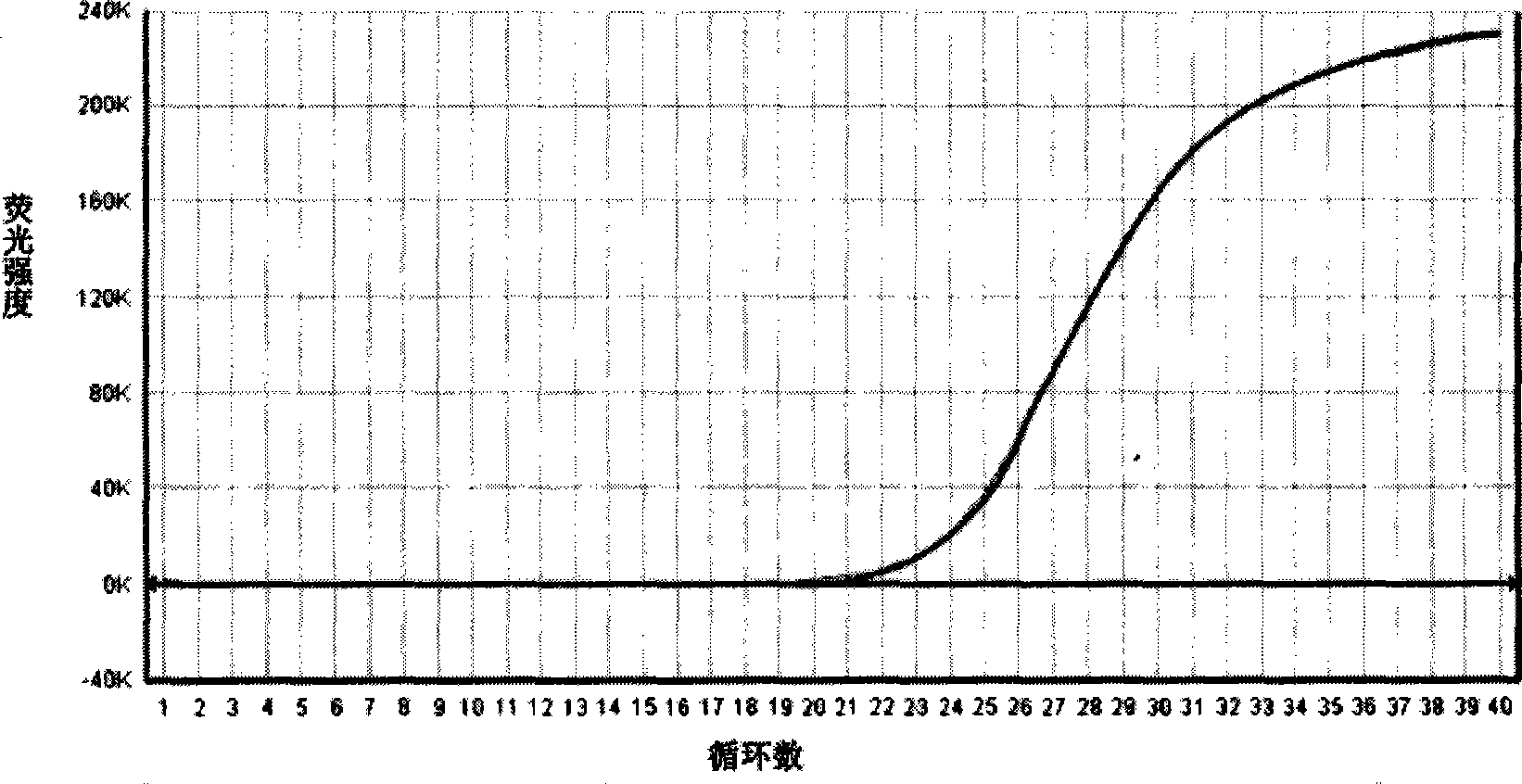
[0026] Weigh tryptone 17g, peptone 3g, sodium dihydrogen phosphate 2.5g, glucose 2.5g, escin 1g, sodium pyruvate 2.0g, mannitol 10g, sodium chloride 25g, lithium chloride 2.5g, distilled water and add to 990ml , heated to dissolve, cooled to room temperature, and corrected the pH value to 7.2-7.3; mixed well, sterilized at 121 °C for 15 min; weighed 15 mg of nalidixic acid and added it to 5 ml of sodium hydroxide solution with a concentration of 0.05 mol / L to obtain nalidixic acid Pyridonic acid solution. Weigh 0.2 mg of potassium tellurite into 5 ml of sterilized distilled water to prepare a potassium tellurite solution. Under aseptic conditions, add 5 ml of nalidixic acid solution and 5 ml of potassium tellurite solution; subpackage and store at 4°C for later use, to prepare a compound enrichment for Salmonella, Listeria monocytogenes and Staphylococcus aureus. culture medium.
[0027] Dilute the prepared medium for Salmonella, Listeria monocytogenes and Staphylococcus aur...
Embodiment 3
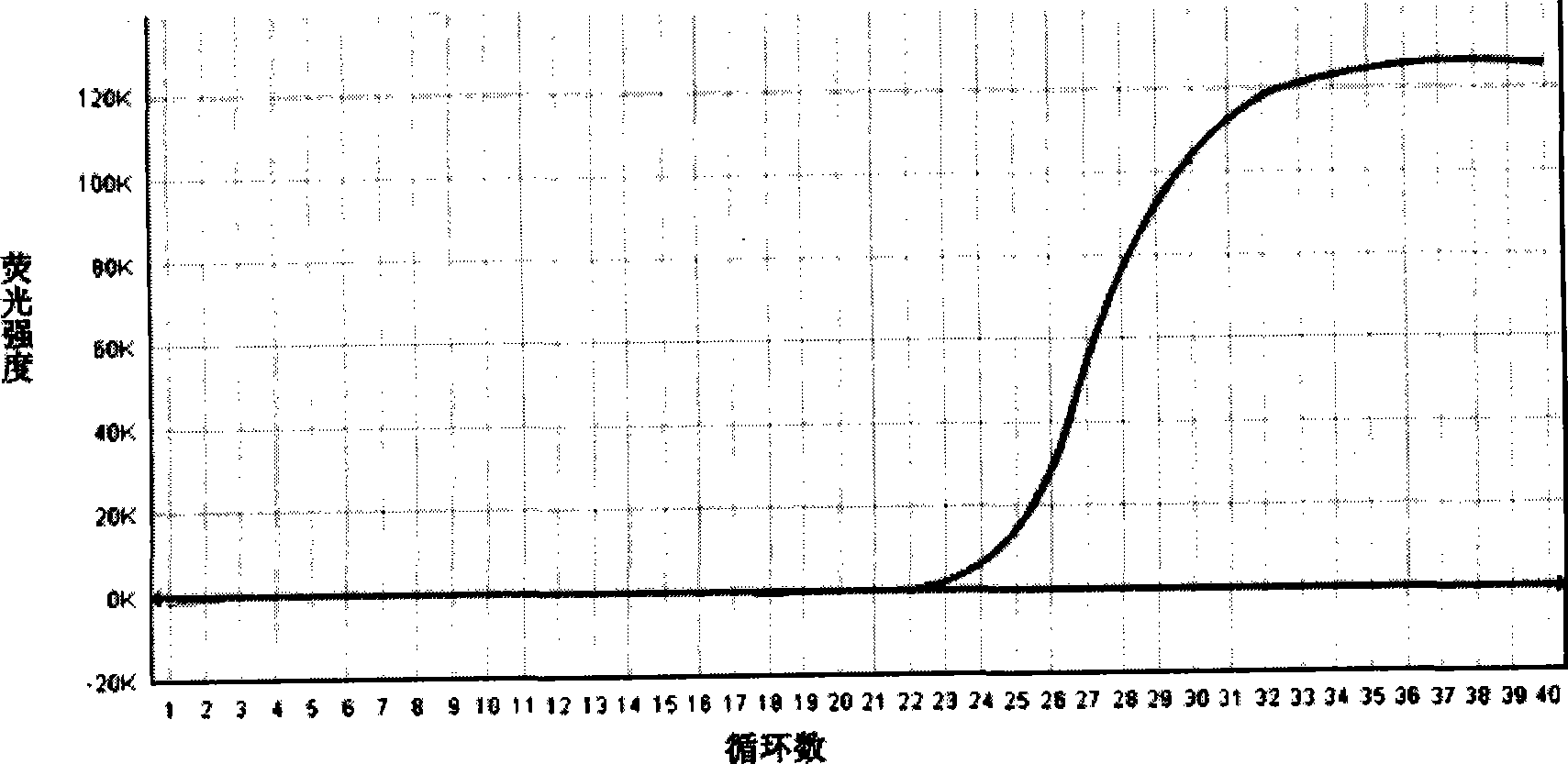
[0032] Weigh tryptone 17g, peptone 3g, sodium dihydrogen phosphate 2.5g, glucose 2.5g, escin 1g, sodium pyruvate 1g, mannitol 3g, sodium chloride 10g, lithium chloride 2.5g, distilled water and add to 990ml, Heat to dissolve, cool to room temperature and correct the pH to 7.2-7.3; mix well, sterilize at 121 °C for 15 minutes; weigh 5 mg of nalidixic acid and add it to 5 ml of sodium hydroxide solution with a concentration of 0.05 mol / L to obtain naphthyridine Keto acid solution. Weigh 0.05 mg of potassium tellurite and add it to 5 ml of sterilized distilled water to prepare a potassium tellurite solution. Under aseptic conditions, add 5 ml of nalidixic acid solution and 5 ml of potassium tellurite solution; subpackage and store at 4°C for later use, to prepare a compound enrichment for Salmonella, Listeria monocytogenes and Staphylococcus aureus. culture medium.
[0033] Dilute the prepared medium for Salmonella, Listeria monocytogenes and Staphylococcus aureus compound enri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com