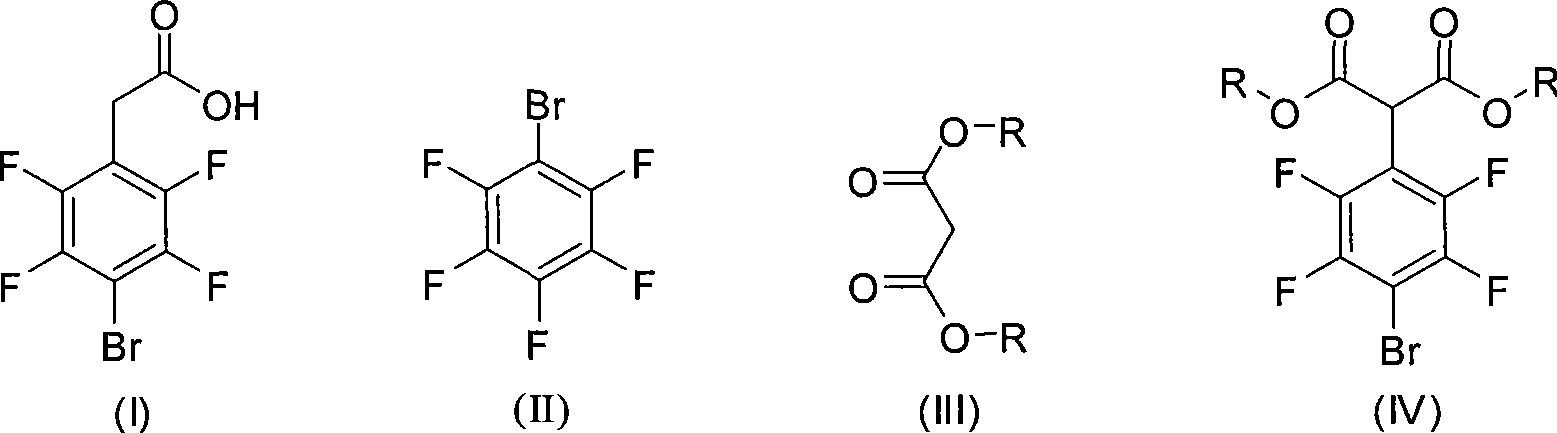
Method for preparing 4-bromo-2,3,5,6-3-fluorophenylacetic acid
A technology of tetrafluorophenylacetic acid and tetrafluorobenzene, applied in the field of 4-bromo-2, can solve the problems of polluting the environment, affecting the health of operators, etc., and achieves the effect of less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
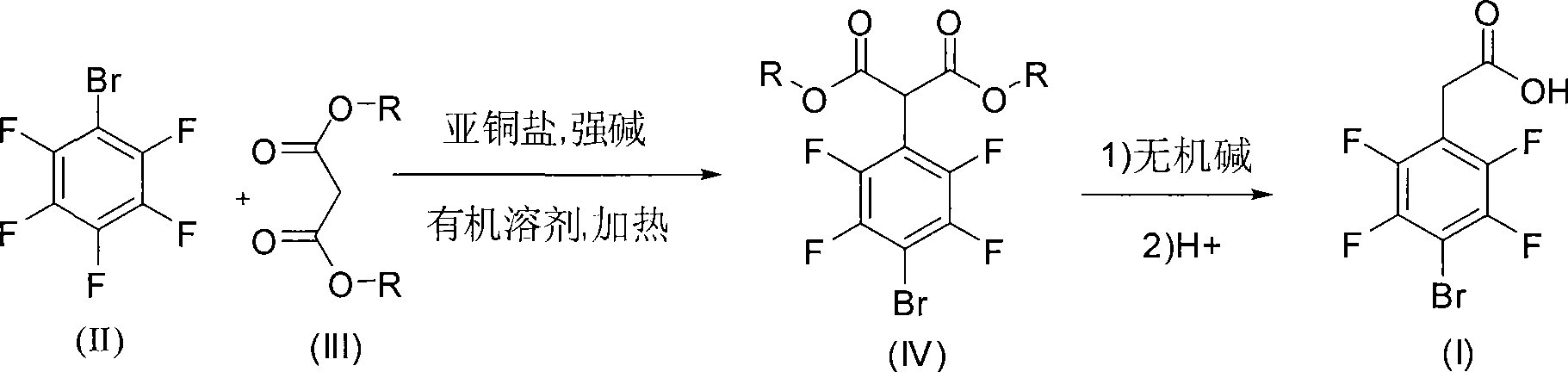
[0030] In a 250ml three-necked flask, add 1,4-dioxane 20ml and potassium tert-butoxide 40mmol (4.12g), N 2 Under protection, 40 mmol (6.4 g) of diethyl malonate and 10 ml of 1,4-dioxane were slowly added dropwise at 50 ° C. After 1 h, the stirring was continued for 0.5 h, and then 4 mmol (0.76 g) of cuprous iodide was added. Then add 20mmol (4.94g) of 2,3,4,5,6-pentafluorobromobenzene, complete the addition in 0.5h, control the temperature to react at 110°C, finish the reaction in 18 hours, wash the reactor with hydrochloric acid and water respectively, Pour into a separatory funnel for liquid separation, extract 2-3 times with ethyl acetate, discard the water phase, evaporate the organic phase to remove the solvent and ethyl acetate under reduced pressure, and obtain a red-colored liquid after refining the residue. 1 H NMR (400MHz, CDCl 3 )δ=1.30(t, J=6Hz, 6H), 4.28(q, J=6Hz, 4H), 4.97(s, 1H). 19 F NMR (376MHz, CDCl 3 ): δ=-135.32--135.21 (m, 2F), -129.23--129.12 (m, 2F).M...
Embodiment 2
[0034] In a 250ml three-necked flask, N 2 Add 4mmol (0.76g) of cuprous iodide and 40ml of 1,4-dioxane under protection. After stirring for 10min, add 40mmol (4.12g) of potassium tert-butoxide, at 50°C N 2 Slowly add 40mmol (6.4g) of diethyl malonate dropwise under protection, and drop it over 2 hours, then add 20mmol (4.94g) of 2,3,4,5,6-pentafluorobromobenzene, react under reflux, and finish in 18 hours For the reaction, wash the reactor with hydrochloric acid and water respectively, pour into a separatory funnel to separate the liquid, extract 2-3 times with ethyl acetate, discard the water phase, and evaporate the organic phase to remove the solvent and ethyl acetate under reduced pressure.
[0035] The remaining liquid from the previous step was added to 5 times of water, 12 g of 40% NaOH was added, and after reflux hydrolysis for 5 hours, the alcohol generated during the hydrolysis process was evaporated under reduced pressure.
[0036] The above solution was extracted w...
Embodiment 3
[0038] Add 30ml of 2-methyltetrahydrofuran and 20mmol of potassium tert-butoxide (2.06g) in a 250ml three-necked flask, 2 Slowly add 20mmol (3.2g) of diethyl malonate and 10ml of 2-methyltetrahydrofuran dropwise under protection. After 1h, add 2mmol (0.38g) of cuprous iodide, and then add 2,3,4,5,6- Pentafluorobromobenzene 10mmol (2.47g), react at 80°C, finish the reaction in 20 hours, wash the reactor with hydrochloric acid and water, separate the liquids in a separatory funnel, extract 2-3 times with ethyl acetate, discard The water phase was removed, and the organic phase was evaporated under reduced pressure to remove the solvent and ethyl acetate.
[0039] The remaining liquid from the previous step was added to 5 times the volume of water, 6 g of 40% NaOH was added, and after reflux hydrolysis for 3 hours, the alcohol generated during the hydrolysis process was evaporated under reduced pressure.
[0040] The above solution was extracted with ethyl acetate, the organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com