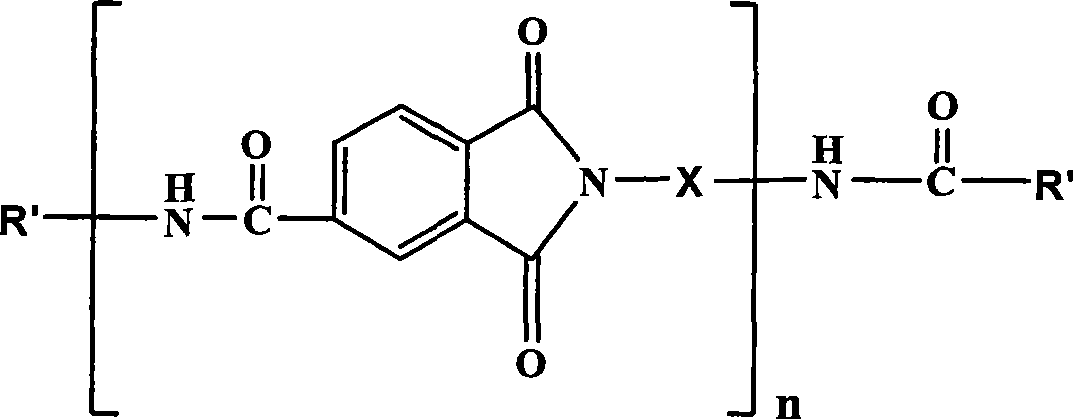
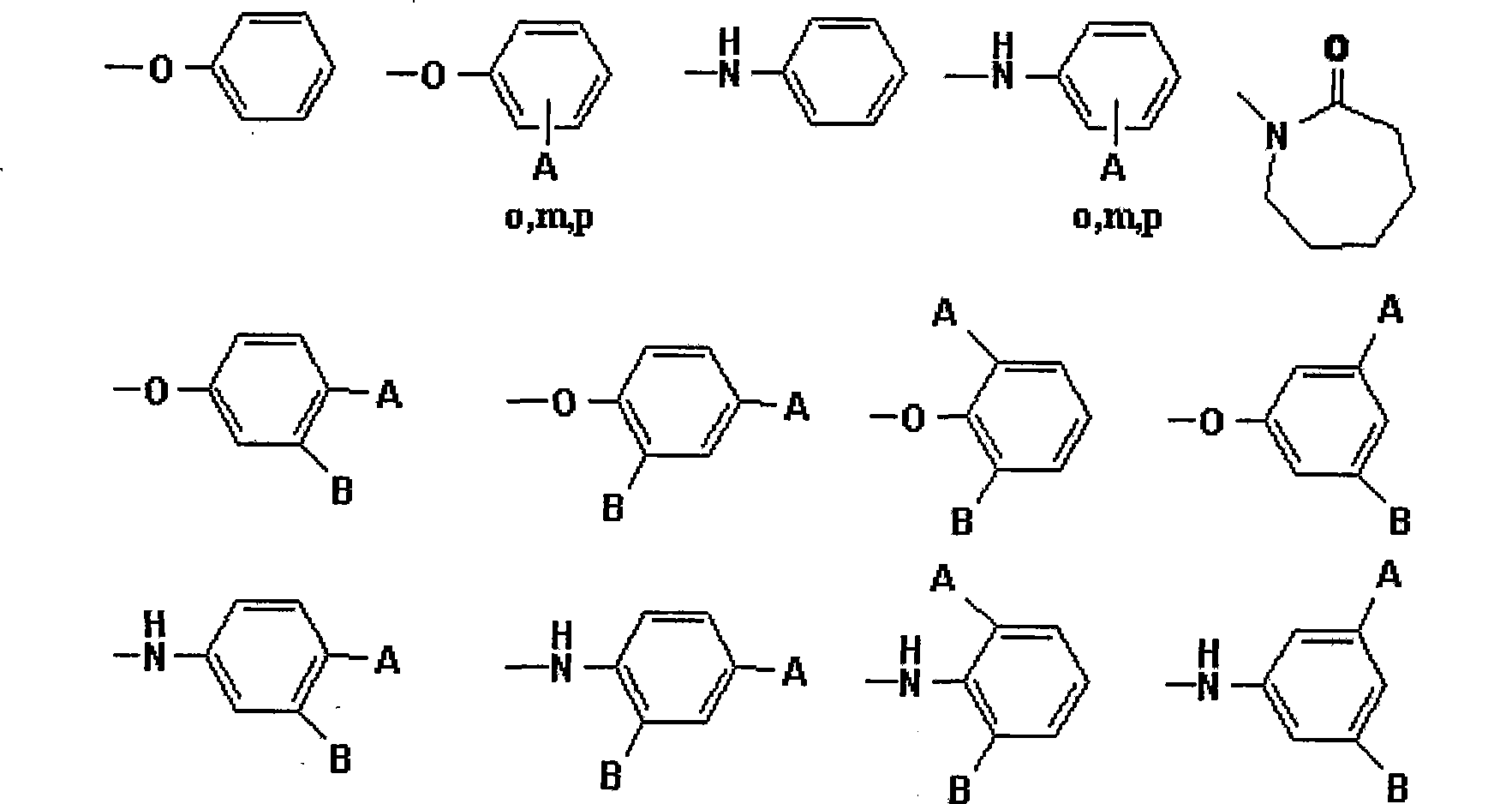
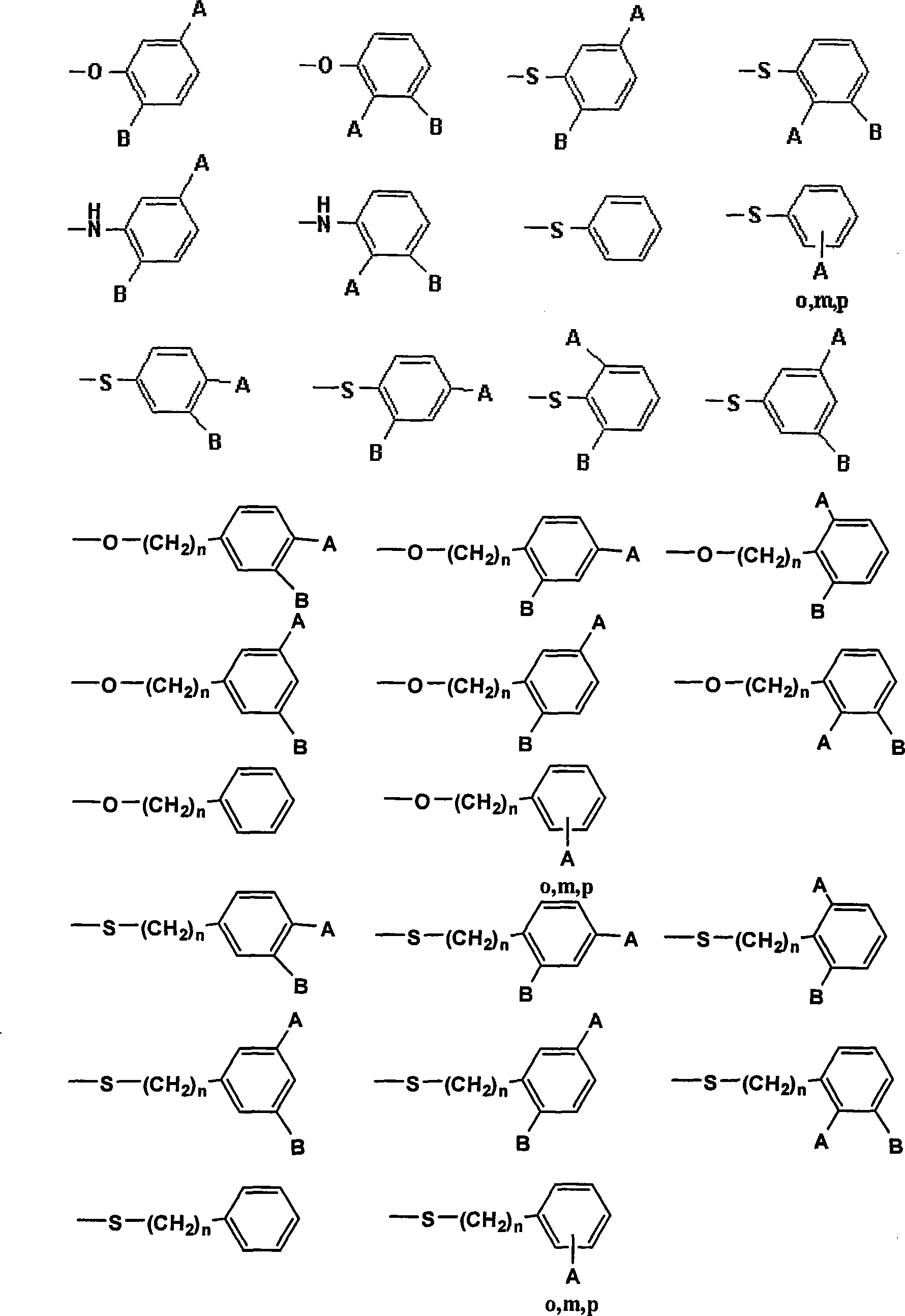
Polyamide acid imide obtained by end-group exchange and preparation thereof
A technology of polyamide-imide and terminal group, which is applied in the field of polyamide-imide and its preparation, can solve the problems of low-molecular-weight end-group-blocked PAI, affect the growth of PAI molecular chain, and limit the application of PAI, so as to broaden the use Range, storage stability, simple polymerization process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 20g (0.0799mol) of diphenylmethane diisocyanate (MDI) and 22.6g (0.176mol) of p-chlorophenol into 120ml of toluene to dissolve, add 0.02g of tri-n-butylamine dropwise under stirring, and then react at a temperature of 40°C After 24 hours, a white precipitate was formed in the system, which was filtered, and the precipitate (referred to as BMDI) was collected and dried.
[0045] Dissolve 1.92g (0.01mol) of trimellitic anhydride with 60ml of N-methylpyrrolidone, then add 10.14g (0.02mol) of BMDI and dropwise add 0.0101g of tri-n-butylamine, then react at 80°C for 0.5h, then raise the temperature to 120°C Continue to react at ℃ for 3h, pour the reaction solution into 2000ml tap water to precipitate, filter to obtain p-chlorophenol-terminated polyamideimide (referred to as PAI-1) and dry it.
[0046] Dissolve 2g of PAI-1 and 0.02g of phenol with 40.4ml of N-methylpyrrolidone, add 0.002g of tri-n-butylamine, then raise the temperature to 80°C for 3 hours, pour the reacti...
Embodiment 2
[0048] Add 12.79g (0.0799mol) of p-phenylene diisocyanate (PPDI) and 24.5g (0.176mol) of p-nitrophenol into 120ml of benzene to dissolve, add 0.1279g of tri-n-butylamine dropwise under stirring, and then at a temperature of 40°C After 20 hours of reaction, a white precipitate was formed in the system, which was filtered, and the precipitate (referred to as BPPDI) was collected and dried.
[0049] Dissolve 1.92g (0.01mol) of trimellitic anhydride with 31ml of N-dimethylformamide (DMF), then add 4.22g (0.01mol) of BPPDI and dropwise add 0.0844g of stannous octoate, then react at 90°C for 1h, then The temperature was raised to 130° C. to continue the reaction for 5 h, the reaction solution was poured into 2000 ml tap water to precipitate, filtered, and p-nitrophenol-terminated polyamideimide (referred to as PAI-1) was obtained and dried.
[0050] Dissolve 2g of PAI-1 and 0.2g of benzyl alcohol in 22ml of N-methylpyrrolidone, add 0.02g of tri-n-butylamine, then raise the temperatu...
Embodiment 3
[0052] Add 20g (0.0799mol) of diphenylmethane diisocyanate (MDI) and 24.5g (0.176mol) of p-nitrophenol into 120ml of toluene to dissolve, add 0.4g of stannous octoate dropwise under stirring, and then react at a temperature of 30°C After 14 hours, a white precipitate was formed in the system, which was filtered, and the precipitate (referred to as BMDI) was collected and dried.
[0053] Dissolve 1.92g (0.01mol) of trimellitic anhydride with 59ml of dimethyl sulfoxide (NMSO), then add 15.85g (0.03mol) of BMDI and dropwise add 0.317g of stannous octoate, then react at 70°C for 2h, then heat up to The reaction was continued at 120°C for 8 hours, the reaction solution was poured into 2000ml of tap water to precipitate, filtered, and p-nitrophenol-terminated polyamideimide (referred to as PAI-1) was obtained and dried.
[0054] Dissolve 2g of PAI-1 and 0.04g of caprolactam in 13.5ml of N,N-dimethylformamide (DMF), add 0.04g of stannous octoate, then raise the temperature to 80°C fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com