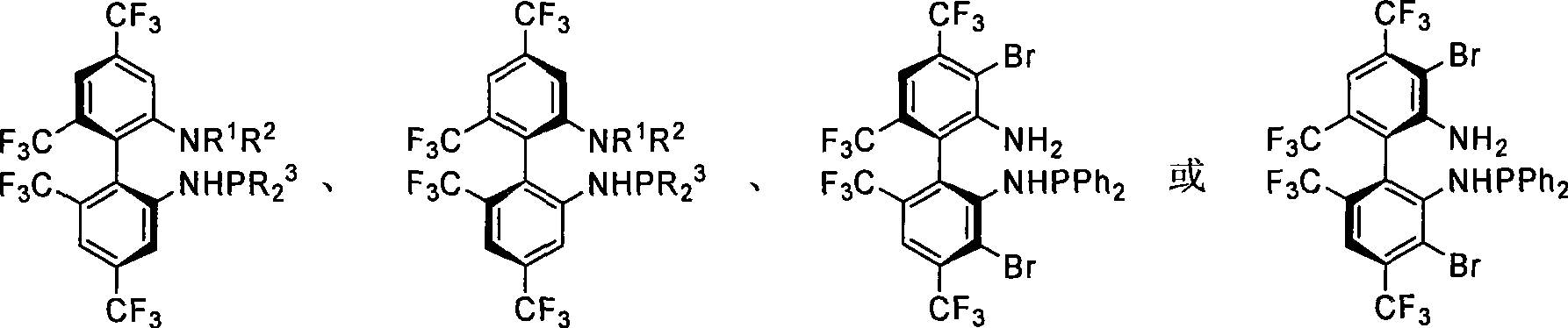
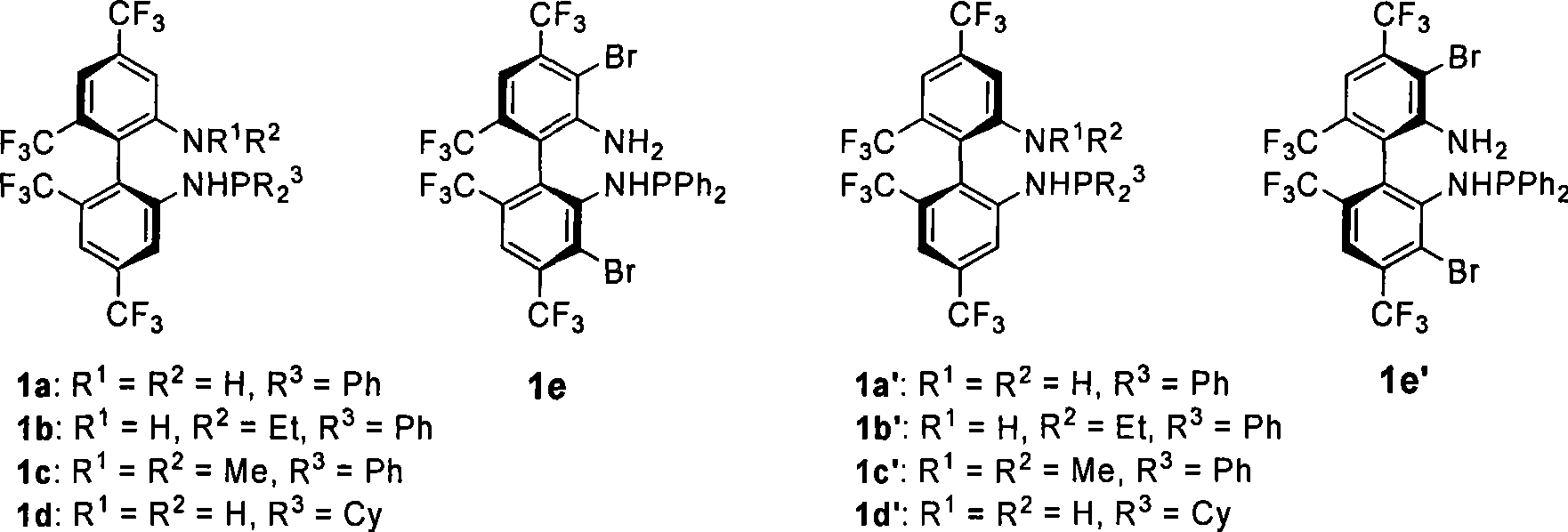
Phosphoramidite monophosphine ligand and preparation method and application thereof
A phosphine amide type, monodentate phosphine ligand technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve the difficulty of monophosphine ligand synthesis And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
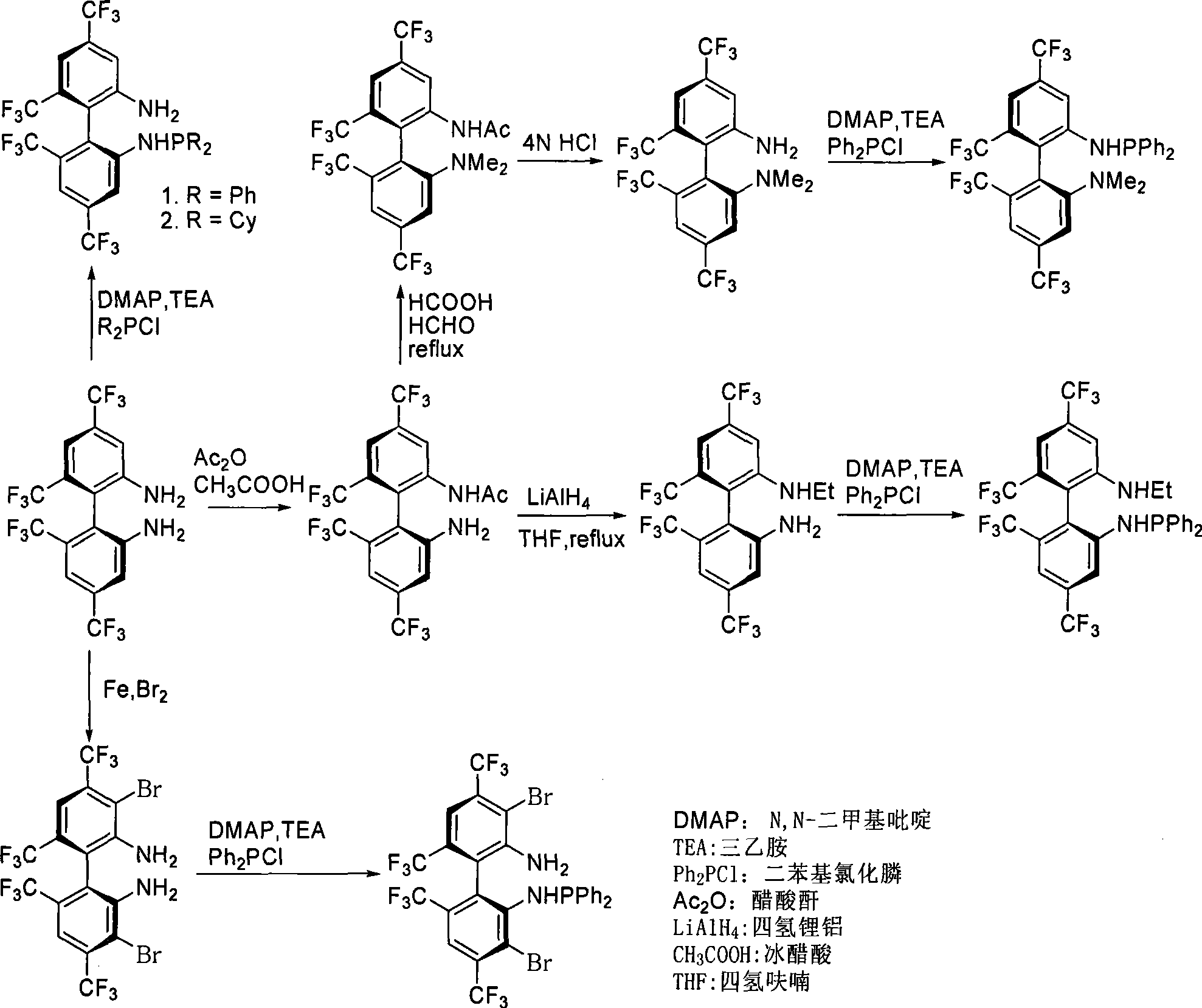
[0020] Preparation of (S)-N-6'-amino-4,6,2',4'-tetrakis(trifluoromethyl)-biphenyl-2-acetamide
[0021] Add (S)-4,4',6,6'-tetrakis(trifluoromethyl)biphenyl-2,2'-diamine (456mg, 1.0mmol) to a 25mL Schlenk bottle at room temperature 10mL of anhydrous dichloromethane, glacial acetic acid (0.6mL, 10.0mmol), acetic anhydride (142μL, 1.5mmol), stirred the reaction mixture overnight, then adjusted the pH to 7-8 with 2N NaOH solution, extracted with dichloromethane , combined the organic phases with saturated NaHCO 3 Washing, saturated brine washing, anhydrous Na 2 SO 4 After drying and removing the solvent, the crude product can be obtained by column chromatography as a white solid (S)-N-6'-amino-4,6,2',4'-tetrakis(trifluoromethyl)-biphenyl-2-ethane Amide 293 mg, yield 56%.
[0022] [α] 25 D =+162.0 (c 0.66, CHCl 3 ); IR(KBr)v 3584, 3431, 1702, 1530, 1491, 1445, 1370, 1276, 1177, 1129cm -1 ; 1 H NMR (CDCl 3 , TMS, 300MHz) 8.86(s, 1H), 7.84(s, 1H), 7.45(s, 1H), 7.27(s, 1H)...
Embodiment 2
[0024] Preparation of (R)-N-6'-amino-4,6,2',4'-tetrakis(trifluoromethyl)-biphenyl-2-acetamide
[0025] Add (R)-4,4',6,6'-tetrakis(trifluoromethyl)biphenyl-2,2'-diamine (456mg, 1.0mmol) to a 25mL Schlenk bottle at room temperature 10mL of anhydrous dichloromethane, glacial acetic acid (0.6mL, 10.0mmol), acetic anhydride (142μL, 1.5mmol), stirred the reaction mixture overnight, then adjusted the pH to 7-8 with 2N NaOH solution, extracted with dichloromethane , combined the organic phases with saturated NaHCO 3 Washing, saturated brine washing, anhydrous Na 2 SO 4 After drying and removing the solvent, the crude product can be obtained by column chromatography as a white solid (R)-N-6'-amino-4,6,2',4'-tetrakis(trifluoromethyl)-biphenyl-2-ethane Amide 306 mg, yield 58.5%.
[0026] [α] 25 D =-161.4 (c 0.66, CHCl 3 ); IR(KBr)v 3584, 3431, 1702, 1530, 1491, 1445, 1370, 1276, 1177, 1129cm -1 ; 1 H NMR (CDCl 3 , TMS, 300MHz) 8.86(s, 1H), 7.84(s, 1H), 7.45(s, 1H), 7.27(s, 1...
Embodiment 3
[0028] Preparation of (S)-N-ethyl-4,4',6,6'-tetrakis(trifluoromethyl)-biphenyl-2,2'-diamine
[0029] Under the condition of Ar, add 5.0 mL of (S)-N-(6′-amino-4,6,2′,4′-tetrakis(trifluoro Methyl)-biphenyl-2-acetamide (270mg, 0.54mmol) in tetrahydrofuran, refluxed for 1.0 hour, carefully added ice-water mixture to the reaction system, extracted unreacted hydride, and then 15% NaOH solution Adjust the pH to 7-8, remove the white solid by filtration, extract with dichloromethane, combine the organic phases with saturated NaHCO 3 Washing, saturated brine washing, anhydrous Na 2 SO 4 After drying and removing the solvent, the crude product can be obtained by column chromatography in yellow oily liquid (S)-N-ethyl-4,4',6,6'-tetrakis(trifluoromethyl)-biphenyl-2,2'- Diamine 190 mg, yield 75.2%.
[0030] [α] 25 D =+131.4 (c 0.72, CHCl 3 ); 1 H NMR (CDCl 3 , TMS, 300MHz) δ 7.42(s, 1H), 7.34(s, 1H), 7.22(s, 1H), 7.09(s, 1H), 3.85(br, 2H), 3.53(br, 1H), 3.20( m, 2H), 1.14(t, J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com