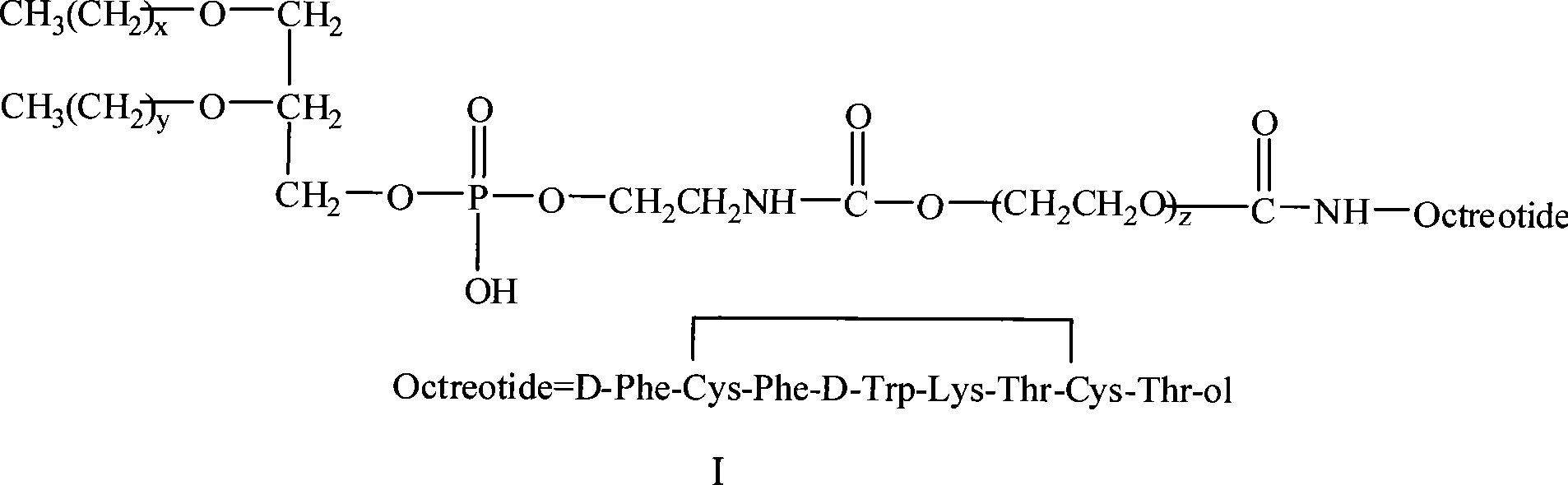PEG-decorated phospholipid derivative using octreotide as target ligand and production method thereof
A polyethylene glycol and octreotide technology, applied in the application of new materials in anti-tumor drug preparations, in the field of polyethylene glycol-modified phospholipid derivatives, can solve the problem of limited space stable immunoliposome development, poor stability, and chemical bond toxicity And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Polyethylene glycol bis-p-nitrophenyl carbonate ((pNP) 2 -PEG) synthesis
[0040] Weigh 2 g of 4-nitrophenyl chloroformate, PEG 4000 20g. The above two substances were dissolved in 4ml and 36ml of anhydrous dichloromethane, respectively, and 1.5ml of triethylamine (TEA) was added to the solution of PEG in anhydrous dichloromethane. The PEG anhydrous dichloromethane solution containing triethylamine was slowly added to the anhydrous dichloromethane solution of 4-nitrophenyl chloroformate under stirring, and the reaction was stirred overnight at room temperature.
[0041] The precipitated triethylamine hydrochloride formed in the reaction solution was filtered off, and the solvent was evaporated to dryness under reduced pressure. The obtained crude product was precipitated with anhydrous ether, and the precipitate was recrystallized twice with ethyl acetate and ether, respectively. After two recrystallizations, the product was dissolved in dichloromethane, and 5 time...
Embodiment 2
[0044] Preparation of p-nitrophenyl carbonate-based polyethylene glycol phosphatidylethanolamine (pNP-PEG-PE)
[0045] Weigh 50mg of distearoylphosphatidylethanolamine (DSPE) and dissolve it in 2.5ml of chloroform, add 0.2ml of TEA, add the above mixture to (pNP) 2 -PEG 4000 Chloroform solution (200mg / ml) 7ml, stirred at room temperature overnight. Chloroform was evaporated to dryness under reduced pressure, 10 ml of 0.01M hydrochloric acid solution was added, and a transparent micellar solution was formed by ultrasonication.
[0046] Gained micellar solution was centrifuged at 10000r / min for 5 minutes, and the supernatant was used for molecular sieve chromatography. Sepharose4B column (2×60cm) was eluted with 0.01M hydrochloric acid (flow rate was 0.35ml / min). The eluate was collected by the partial collector, and the ultraviolet absorption of each part was measured at 270nm. Plot the elution profile. The molybdenum blue color reaction detected by TCL is the position of p...
Embodiment 3
[0049] Linkage of pNP-PEG-PE to Octreotide
[0050] pNP-PEG 4000 - Dissolve 50mg of PE in chloroform, remove chloroform under reduced pressure to form a lipid film, dissolve 10mg of octreotide in 0.01mol / l HCl 4ml, add to the lipid film, shake gently to fully disperse the lipid film. Add 20ml of 10mmol / l (pH9.0) Tris buffer to the above suspension, mix well, and react overnight at 4°C. Using water as the medium, the dialysis bag with a molecular mass of 3kD was dialyzed for 2 days, and the product was freeze-dried.
[0051] 1 H-NMR (300MHz, D 2 O): δ 0.85-0.89 (CH 3 -), δ 1.25 (-CH 2 -), δ 2.28-2.40(-OC H 2 C H 2 NH-), δ 3.60-3.73 (O-CH 2 CH 2 -O), δ 7.10-7.35 (-NHCO-).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com