Phloroglucine orally disintegrating tablet and preparation method thereof
A technology of orally disintegrating tablets and phloroglucinol, which is applied in the direction of pill delivery, effective ingredients of hydroxyl compounds, antipyretics, etc., can solve the problem of high process conditions and equipment requirements, inconvenient packaging, storage and transportation, and difficulty in ensuring the integrity of the appearance In order to achieve the effect of simple process conditions and equipment requirements, improve bioavailability, and maintain the integrity of appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
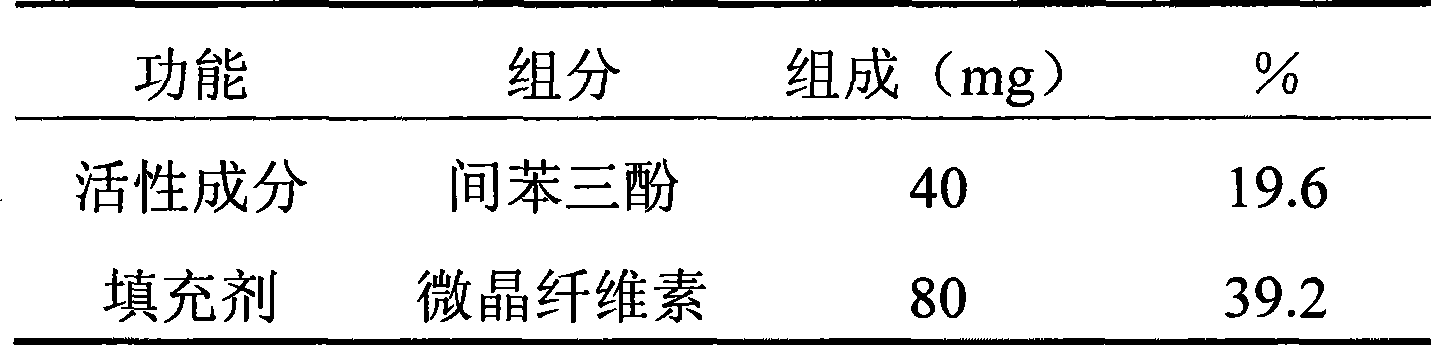
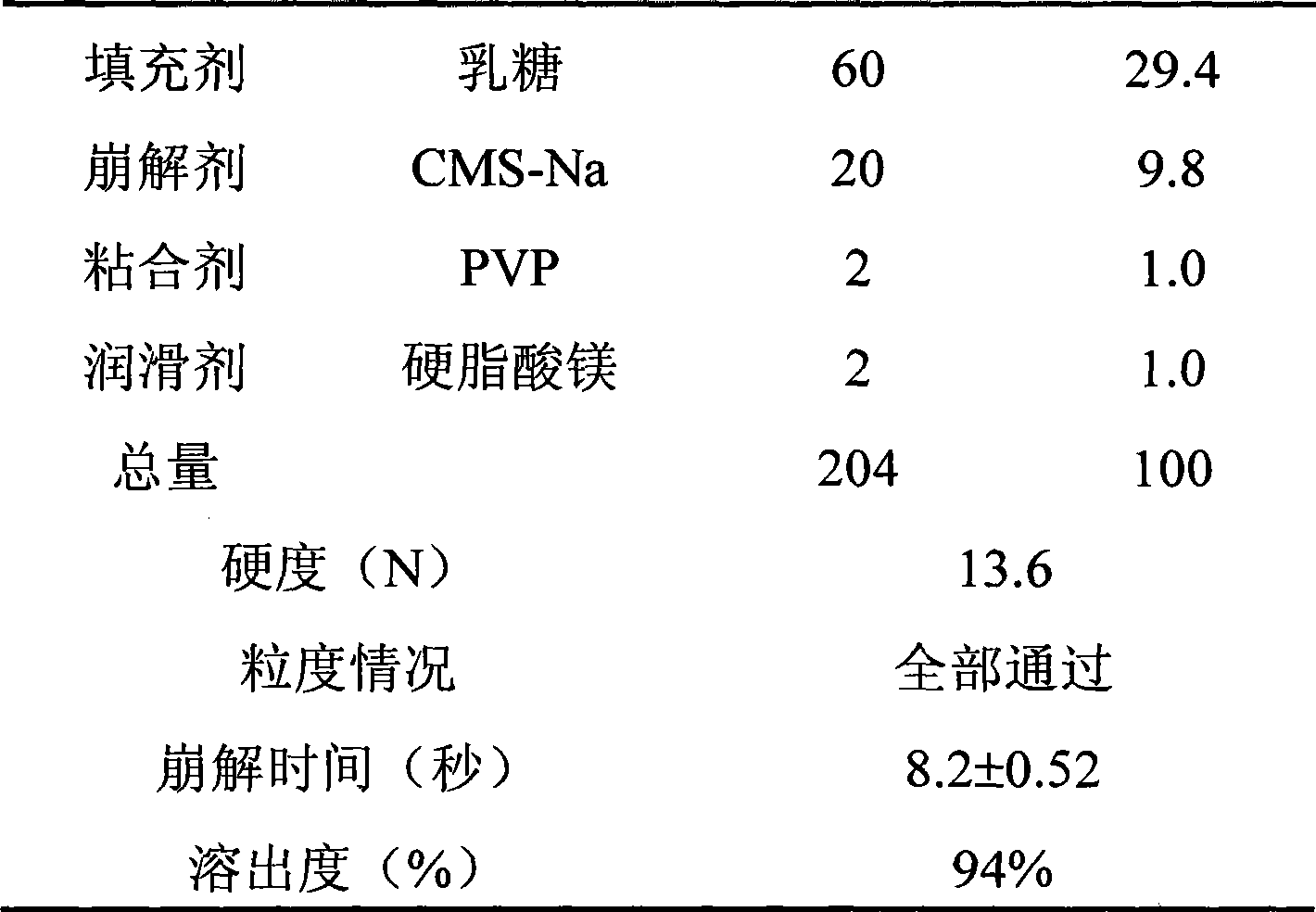
[0043] Weigh each component according to the table below, and mix evenly the phloroglucinol and the auxiliary materials except the binder and the lubricant according to the method of equal increment. Pass the resulting mixture through a 40 mesh screen twice and place the resulting material in a suitable container. A 5% (g / 100ml) PVP aqueous solution of the binder was added to the container to make a soft material. The soft material was extruded through a 20 mesh screen to produce wet granules. The wet granules were placed in a dry enamel tray, and put together into a forced air constant temperature drying oven, and dried at 50° C. for 2 hours. Weigh the weight of the dry granules, add a lubricant, mix well, and press into tablets with a tablet machine to obtain orally disintegrating tablets containing phloroglucinol.
[0044] The hardness of the tablet was measured with a hardness tester, and the particle size, disintegration time and dissolution rate of the prepared orally ...
Embodiment 2
[0055] The preparation method is the same as in Example 1, and the adhesive is prepared as a 10% (g / 100ml) aqueous solution for use. The components and measurement results of the phloroglucinol orally disintegrating tablet are shown below.
[0056]
[0057]
Embodiment 3
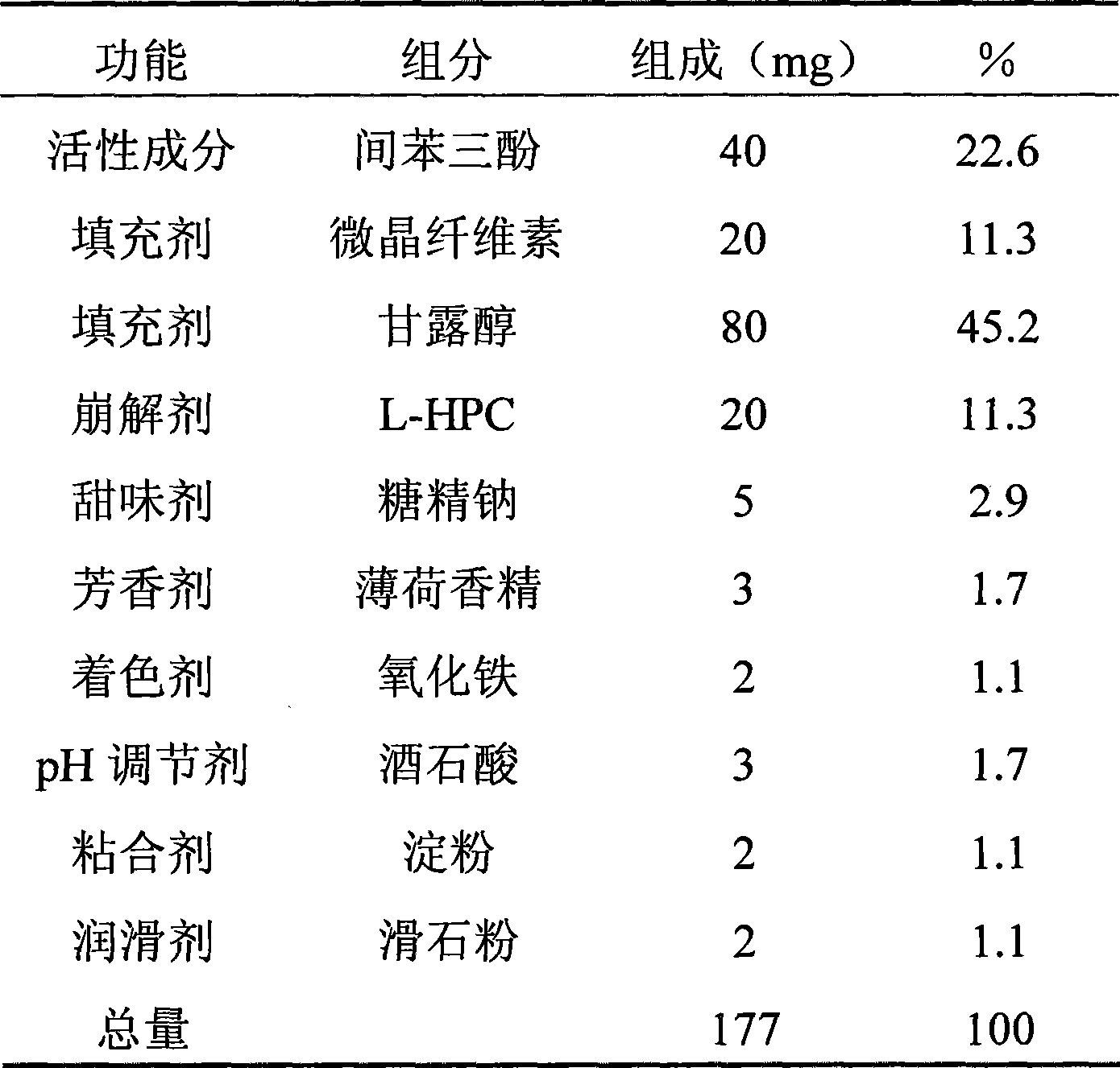
[0059] The preparation method is the same as in Example 1, and the components and measurement results of the phloroglucinol orally disintegrating tablet are shown below.
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com