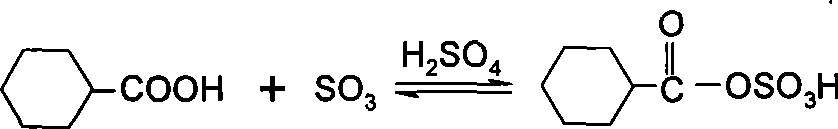Method for preparing caprolactam by nitrosation reaction
A caprolactam and nitrosation technology, applied in the preparation of lactam, chemical instruments and methods, organic chemistry, etc., to achieve high conversion rate and selectivity, improve selectivity, and avoid the effect of vapor-liquid entrainment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
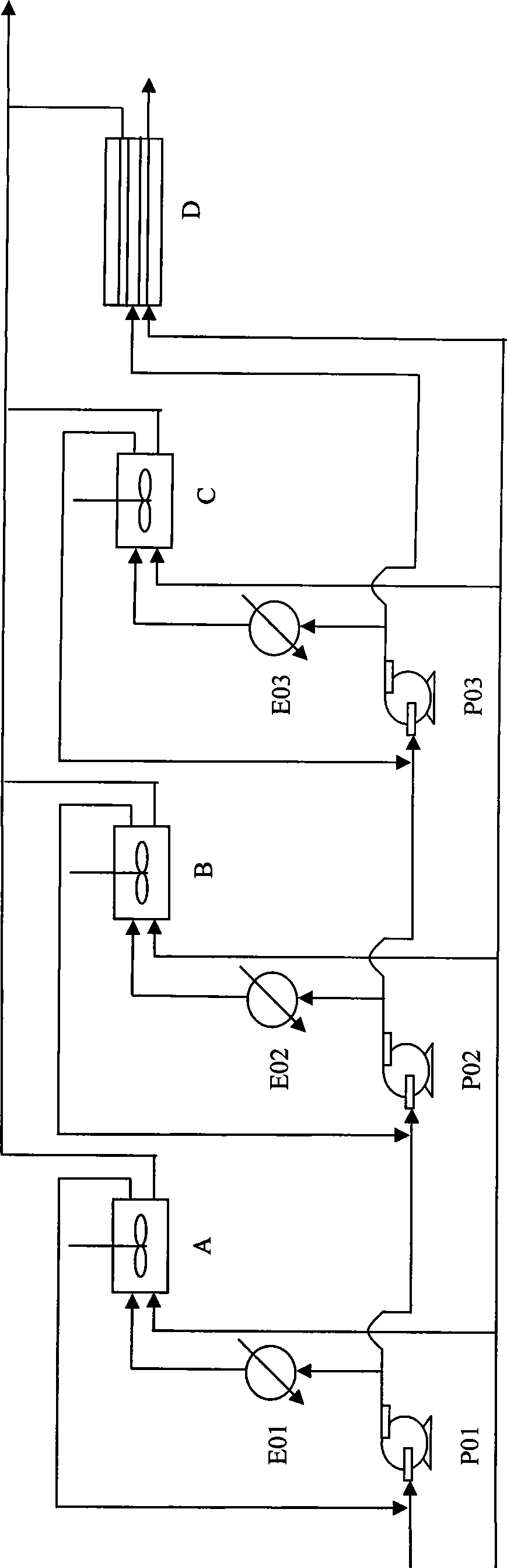
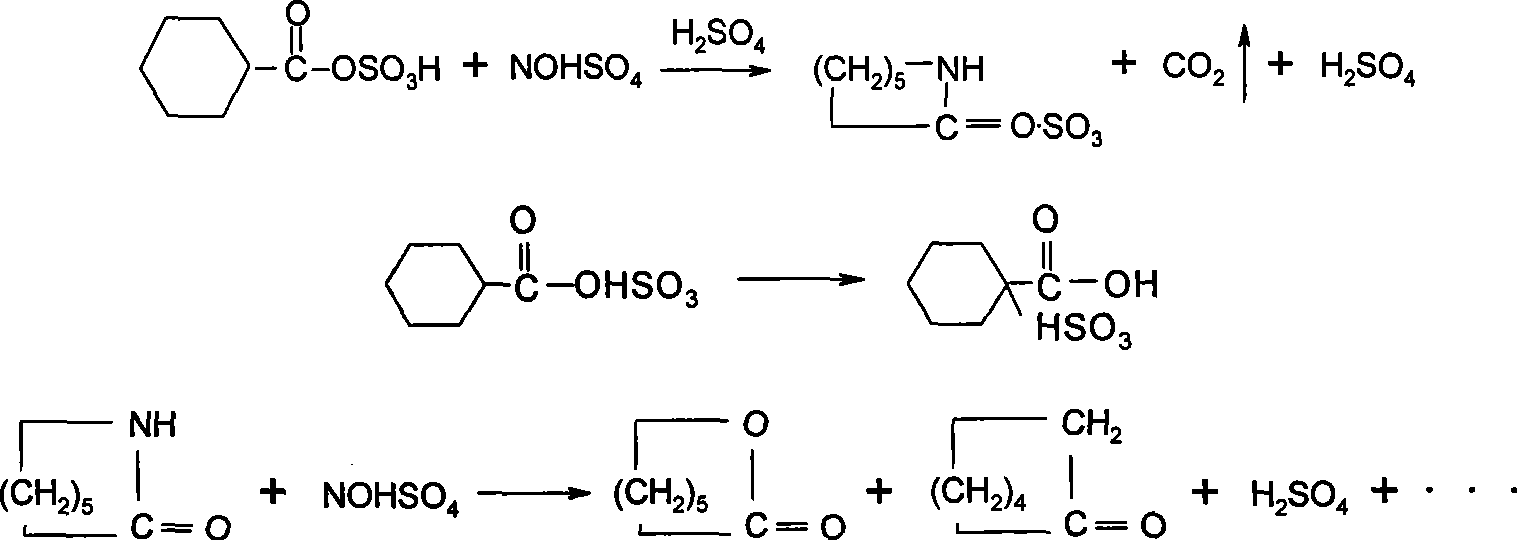
[0061] Embodiment 1: get 10.0g mass percent concentration and be the cyclohexyl formic acid of 99.19%, add 6.8g free SO 3 The concentration is 41.21% of fuming sulfuric acid to react to obtain mixed anhydrides. Under normal pressure, 6.2g of nitrosyl sulfuric acid with a mass percentage concentration of 73.54% was added dropwise to the mixed acid anhydride to carry out the nitrosation reaction. After quenching in a water bath, n-hexane was added, and 9.6 g of water was added to carry out the hydrolysis reaction, and the hydrolysis temperature was controlled not to exceed 30°C. After standing still, 26.0 g of the heavy phase product and 118.9 g of the light phase product were separated. The concentration of caprolactam in the heavy phase was 13.23% by liquid chromatography analysis, and 4.81% of cyclohexyl formic acid in the light phase was obtained by titration analysis. As shown in Table 2, the conversion rate of nitrosation reaction is 42.34%, the yield of caprolactam is 39...
Embodiment 2
[0062] Embodiment 2: Raw material proportioning, feeding method is the same as that of Embodiment 1, the difference lies in the dropping time and reaction time, and the conversion rate, yield and selectivity of nitrosation reaction are shown in Table 2.
Embodiment 3~4
[0063] Embodiments 3 to 4: The feeding method is the same as that of Embodiment 2, except that the concentration of oleum is 39.47%, the time for dropping and the temperature rise of the reaction, and the conversion rate, yield and selectivity of the nitrosation reaction are shown in Table 2.
[0064] Table 2 Results of nitrosation reaction under solvent-free conditions
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com