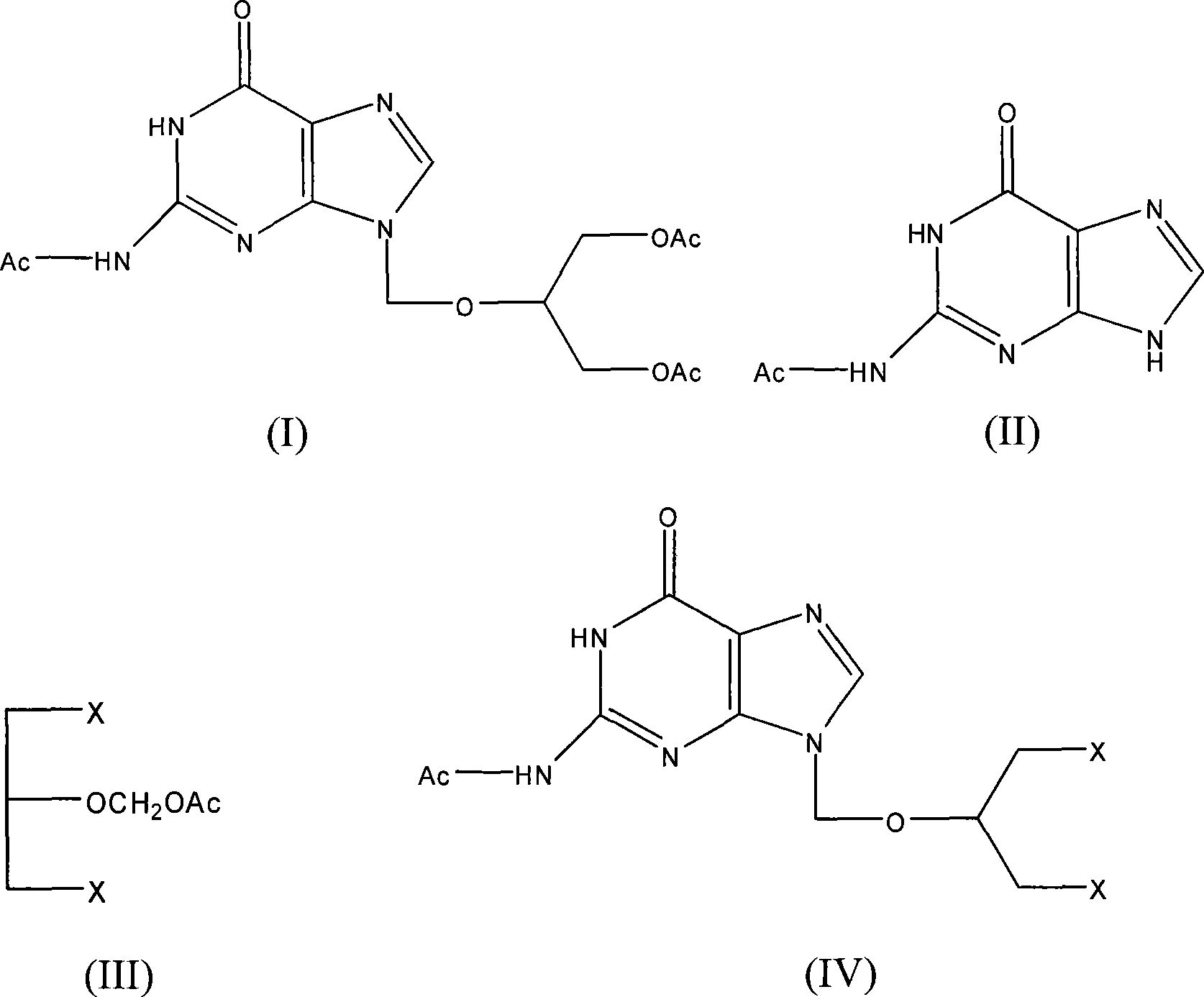
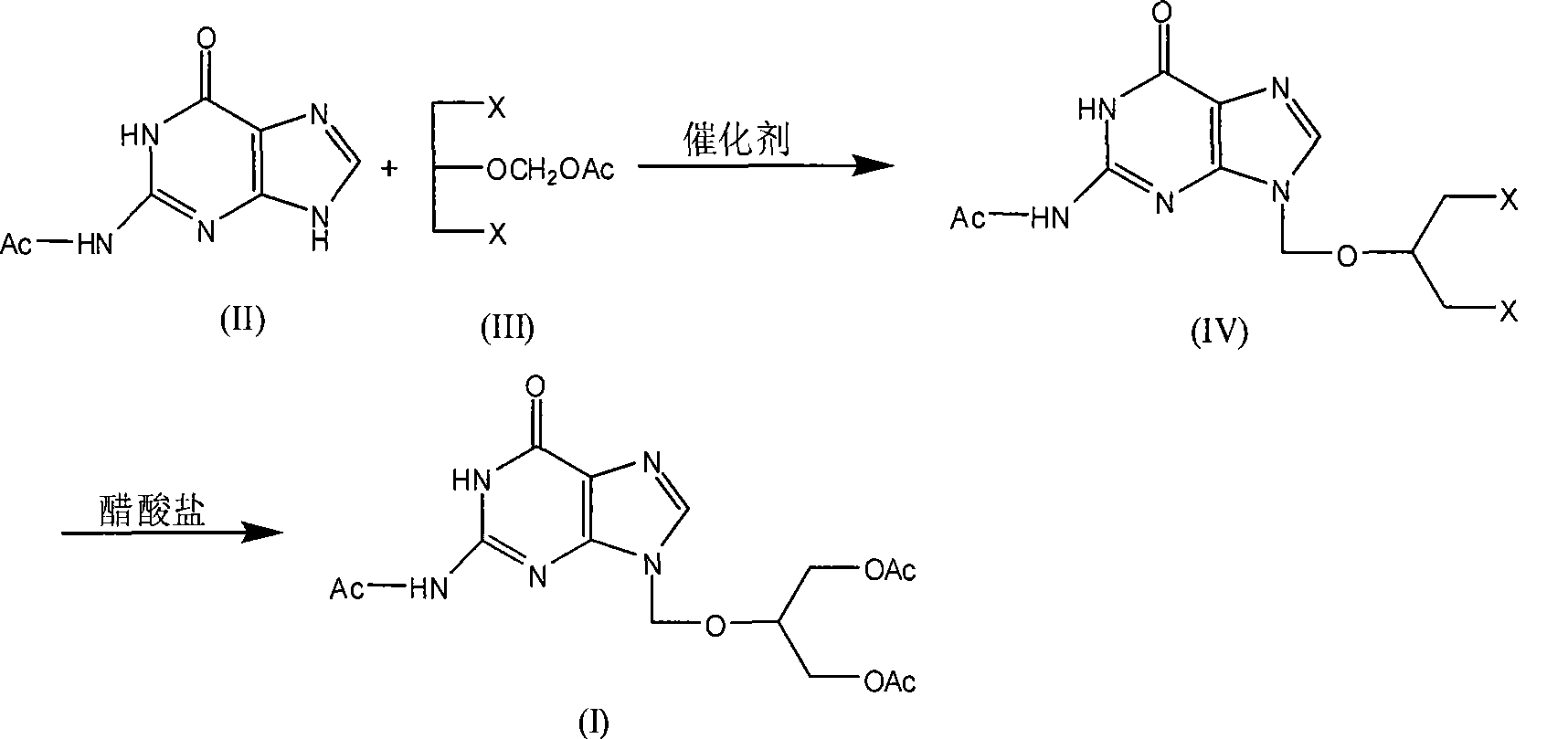
Chemical synthesis of triacetylganciclovir
A technology of chemical synthesis and ganciclovir, applied in the field of chemical synthesis of triacetyl ganciclovir, can solve the problems of many other impurities, inability to carry out refining and purification, and need to be refined for about 8 times to be barely qualified, and achieve selectivity. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Feeding molar ratio monoacetylguanine: 1,3-dichloro-2-(acetoxymethoxy) propane: catalyst is 1:1.5:0.03, catalyst is p-toluenesulfonic acid, organic solvent is toluene, and its consumption is 5 times the weight of monoacetylguanine.
[0034] In a 250ml four-necked flask equipped with a thermometer, a reflux condenser, a constant pressure dropping funnel, and a mechanical stirrer, add 19.3g (100mmol) of monoacetylguanine, 1,3-dichloro-2-(acetoxymethoxy base) propane 30.2g (150mmol), toluene 96.5g and catalyst 0.58g. After the addition, heat up to 100°C, and react at 100°C for 48 hours, followed by TLC monitoring (developing solvent: chloroform-methanol-ammonia (8:2:0.2)), after the reaction is complete, stop heating, cool naturally, and cool with ice-salt water. Temperature 0°C, filter with suction, wash the filter cake with 20ml of acetone, put the solid into a three-neck flask, soak and stir with 80ml of acetone for 10 minutes, filter with suction, and dry the filter c...
Embodiment 2
[0049] Feeding molar ratio monoacetylguanine: 1,3-dichloro-2-(acetoxymethoxy)propane: catalyst is 1:1.1:0.015, catalyst is concentrated sulfuric acid, organic solvent is xylene, and its consumption is mono 5 times the weight of acetylguanine.
[0050] In a 250ml four-necked flask equipped with a thermometer, a reflux condenser, a constant pressure dropping funnel, and a mechanical stirrer, add 19.3g (100mmol) of monoacetylguanine, 1,3-dichloro-2-(acetoxymethoxy base) propane 22.1 g (110 mmol), xylene 96.5 g and catalyst 0.15 g. After the addition, heat up to 120°C, and react at 120°C for 24 hours, followed by TLC monitoring (developing solvent: chloroform-methanol-ammonia (8:2:0.2)), after the reaction is complete, stop heating, cool naturally, and cool with ice-salt water. The temperature is 0°C, suction filtration, the filter cake is washed with 20ml of acetone, the solid is put into a three-neck flask, soaked with 80ml of acetone and stirred for 10 minutes, suction filtrat...
Embodiment 3
[0065] Feeding molar ratio monoacetylguanine: 1,3-dichloro-2-(acetoxymethoxy) propane: catalyst is 1:1.5:0.03, catalyst is p-toluenesulfonic acid, organic solvent is DMF, and its consumption is 5 times the weight of monoacetylguanine.
[0066] In a 250ml four-necked flask equipped with a thermometer, a reflux condenser, a constant pressure dropping funnel, and a mechanical stirrer, add 19.3g (100mmol) of monoacetylguanine, 1,3-dichloro-2-(acetoxymethoxy base) propane 30.2g (150mmol), DMF96.5g and catalyst 0.58g. After the addition, heat up to 150°C, and react at 150°C for 48 hours, followed by TLC monitoring (developing solvent: chloroform-methanol-ammonia (8:2:0.2)), after the reaction is complete, stop heating, cool naturally, and cool with ice-salt water. The temperature is 0°C, suction filtration, the filter cake is washed with 20ml of acetone, the solid is put into a three-neck flask, washed with 80ml of acetone and stirred for 10 minutes, suction filtered, the filter ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com