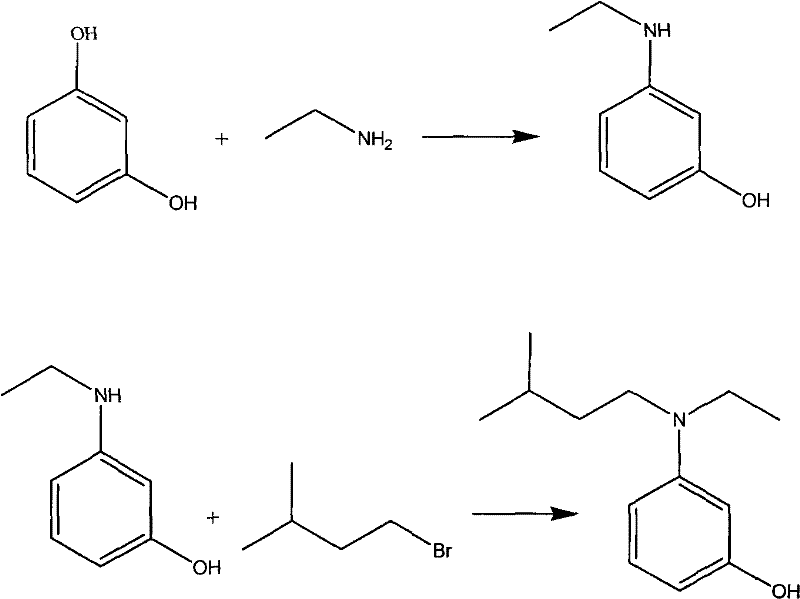
Preparation of 3-(N-ethyl-N-isoamyl) amino phenol
A technology of ethylaminophenol and aminophenol, which is applied in the field of preparation of 3-aminophenol, can solve the problems of high production process cost, unfavorable industrial production, and low purity of EPA, and achieve the effects of low price, easy industrial production, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1, the preparation method of 3-(N-ethyl-N-isoamyl) aminophenol is that at room temperature, successively 77g resorcinol and 53g content are 65% monoethylamine aqueous solution to add 250ml In a high-pressure reactor, heat up to 165-175°C, keep warm until the reaction is over, transfer the material to a beaker when the temperature drops to 40°C after the reaction, and slowly add 80ml of 30% liquid caustic soda under stirring, that is, containing hydrogen Sodium 0.8mol, add 25g caustic soda after adding, continue to cool down to 0-10°C, filter to get about 160g of 3-ethylaminophenol sodium salt; add 50ml water into a 1000ml flask at room temperature, add 3-ethylaminophenol under stirring 160g of sodium phenolate, adjust the pH to 6-7 with industrial hydrochloric acid, then raise the temperature to 80°C, add 95g of isopentyl bromide dropwise, and keep it warm for reaction. About 100 g of the product 3-(N-ethyl-N-isoamyl)aminophenol was obtained, with a content o...
Embodiment 2
[0014] Embodiment 2, the preparation method of 3-(N-ethyl-N-isoamyl) aminophenol is at room temperature, successively 77g resorcinol and 58g content are 60% ethylamine aqueous solution to add 250ml high pressure In the reaction kettle, heat up to 180°C and keep warm until the reaction is over. When the material cools down to 30°C after the reaction, transfer it to a beaker, slowly add 100ml of 30% liquid caustic soda under stirring, and then add 30g of caustic soda , continue to cool down to 0-10°C, filter to obtain about 160g of 3-ethylaminophenol sodium salt; add 50ml of water into a 1000ml flask at room temperature, add 160g of the previous reaction product 3-ethylaminophenol sodium salt under stirring, and use industrial Adjust the pH to 6-7 with hydrochloric acid, then raise the temperature to 80°C, add 150g of bromoisopentane dropwise, and keep it warm for reaction. Ethyl-N-isoamyl)aminophenol is about 100g, and the content is more than 95.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com