Inhibitors of plasma kallikrein
A compound, selected technology, applied in medical preparations containing active ingredients, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve problems such as short half-life, limited to acute research, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Synthesis of PK-specific inhibitors
[0157] Using PS-carbodiimide (obtained from Biotage, Inc., Charlottesville, Virginia) and the synthetic scheme provided by Biotage (see Figure 1), the Carboxylic acid (from ASDI Co., Ltd., Kyiv, Ukraine, or Enamine Co., Ltd.) and 4-amidino-benzylamine (4-AmBz, from Astarte, Bristol, Pennsylvania) Co., Ltd. (Astatech, Inc.)) to form an amide bond to synthesize the compound of the present invention. For each synthesis, 100 micromoles of PS-carbon Diimine with a carboxylic acid (75 μmol), 4-AmBz.2HCl (75 μmol), N-hydroxybenzotriazole (HOBt; 75 μmol) and diisopropylethylamine (DIPEA; 75 micromoles) and mixed on an Adams Nutator (Adams Nutator) at room temperature for 48-72 hours. The product was obtained by filtration, then evaporated to remove DCM. The residual amount of uncoupled 4-AmBZ was determined by quantification of free amine functional groups on 4-AmBZ with ninhydrin, whereby it was determined that more than 95% of the carbo...
Embodiment 2
[0159] Example 2: Research on the inhibitory effect of PK inhibitors
[0160] Human plasma kallikrein (PK) was obtained from Haemtech Technologies, Essex Junction, Vermont. The enzymatic activity of PK was measured with synthetic peptide substrate H-D-Pro-Phe-Arg-pNA (Bachem, Inc., Switzerland), and the cleavage of the substrate by the enzyme would result in A 405 Elevated, which can be used by Molecular Devices (Molecular Devices) V max Dynamic microplate reader to measure. In each well of a microplate reader plate, add 190 μl of PK solution (1 nM in 0.05M HEPES, pH 7.5, 0.01% Triton X-100) to 10 μl of H-D-Pro-Phe-Arg-pNA (2mM, contained in DMSO), shake and mix immediately, measure A in 120-180 seconds 405 The rate of increase of , thus determining the uninhibited PK activity (control). In a parallel experiment, the compound of the present invention was mixed with the synthetic substrate in each well to achieve a final concentration of 0.01-10 micromolar, the volume of th...
Embodiment 3
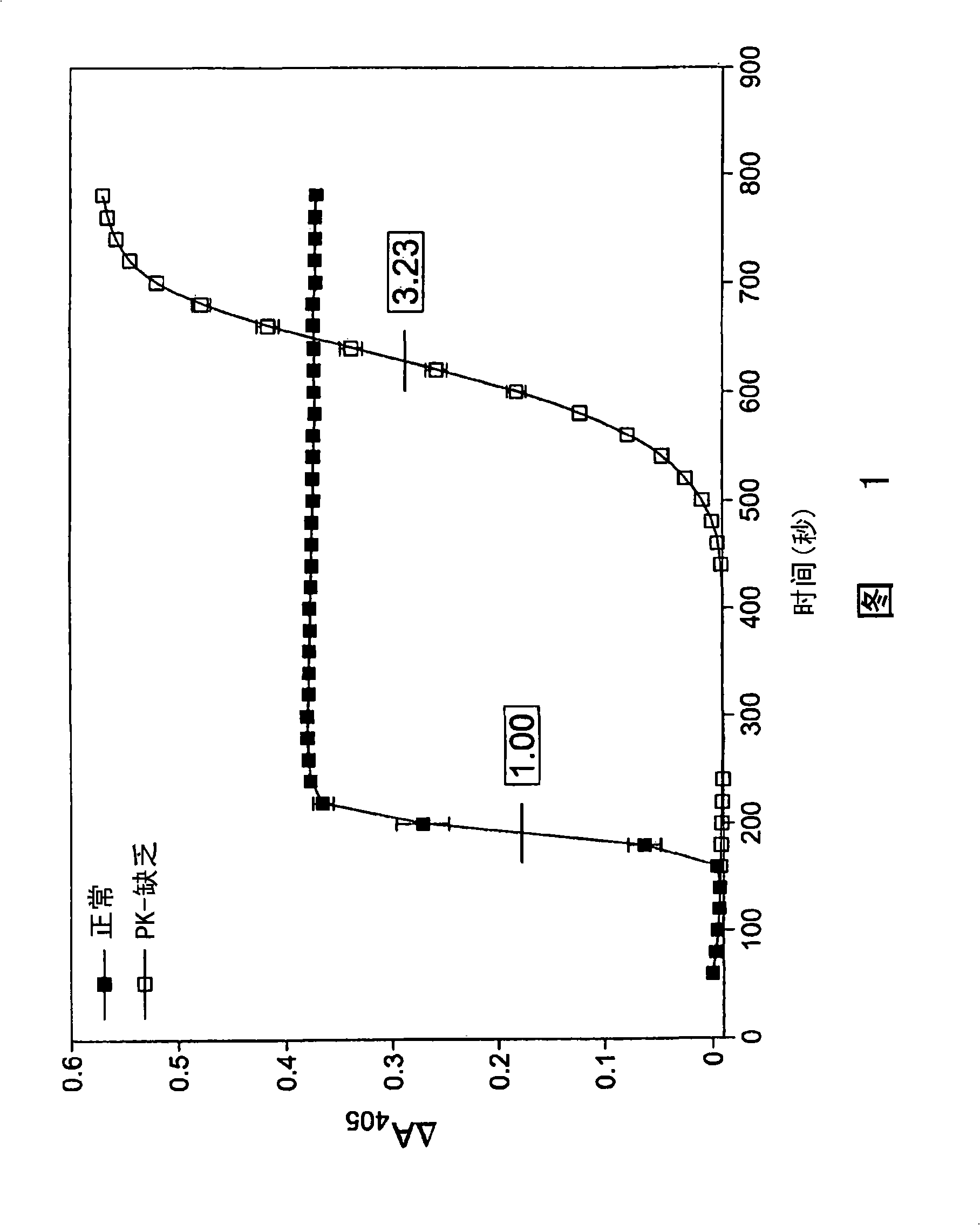
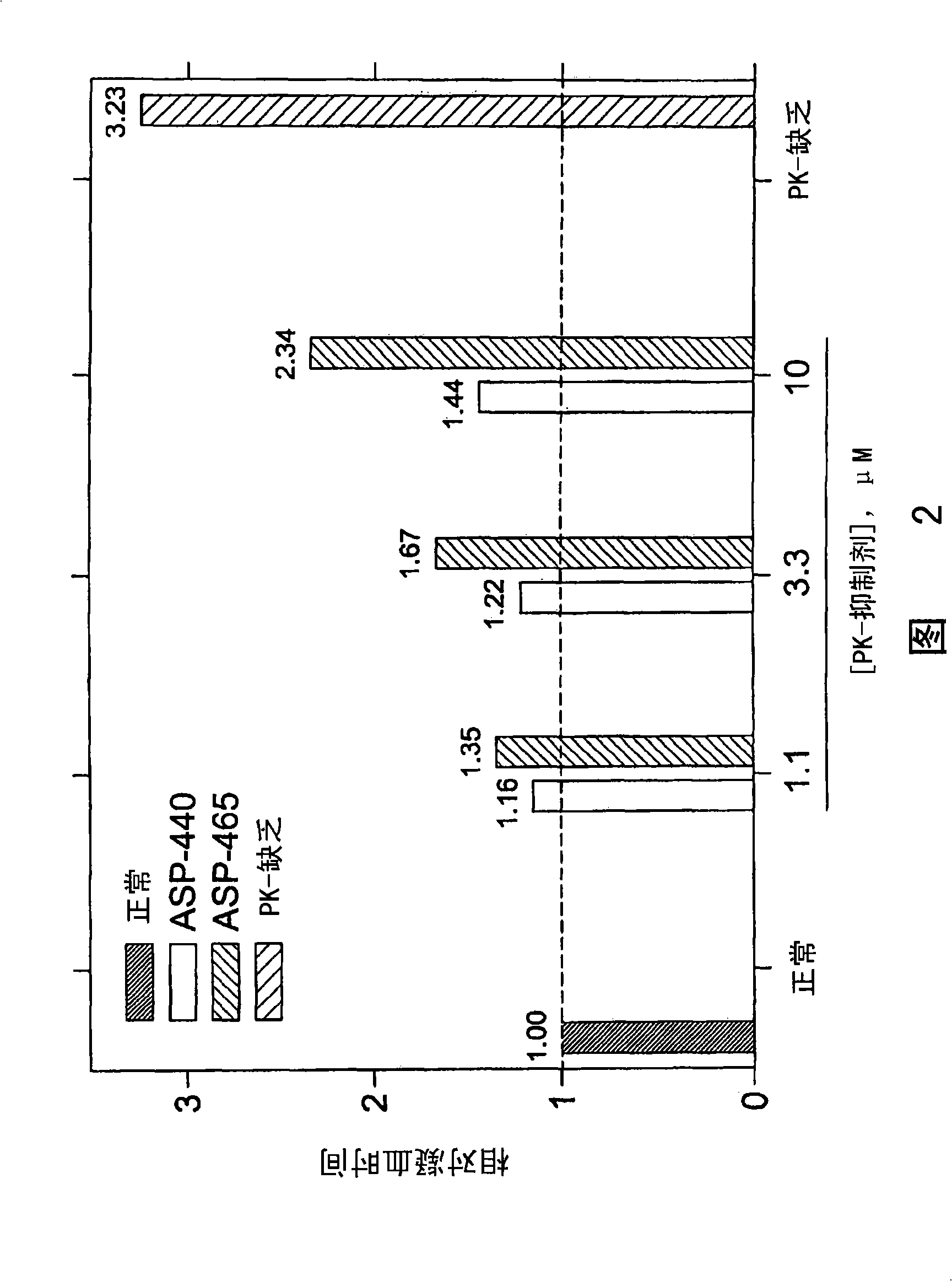
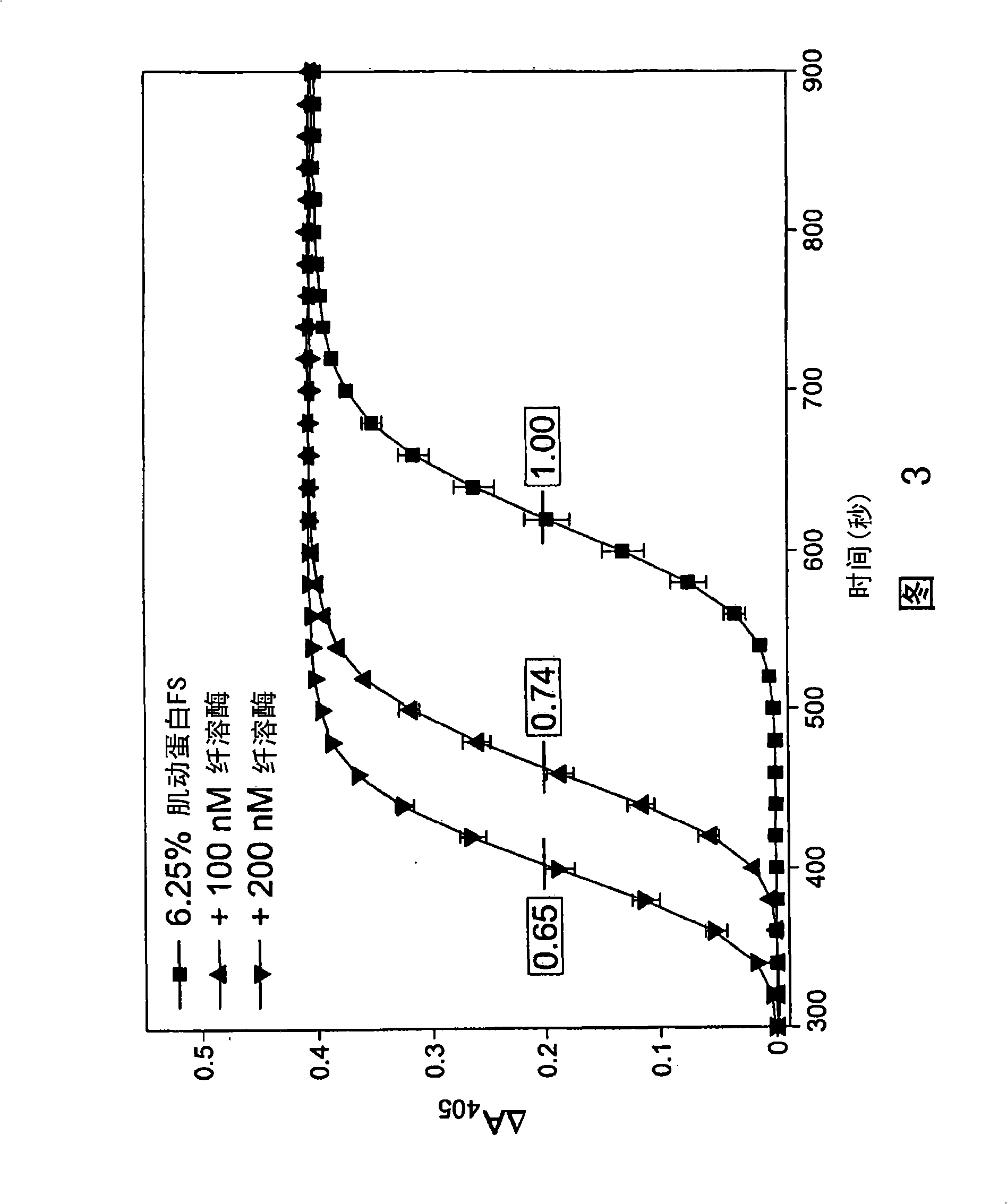
[0175] Determination of anticoagulant activity of PK inhibitors
[0176] To determine the role of PK on thrombin generation, fibrin cleavage, and ultimately fibrin clot formation upon activation of the intrinsic pathway, contact activation of the intrinsic pathway was initiated using commercially available Actin FS reagent (Dade-Behring) . Human plasma (50 μl) was mixed with 15 mM CaCl in each well of a clean polystyrene 96-well microplate. 2 Actin FS (50 μl) was mixed, passed through a dynamic microplate reader (Molecular Devices (Molecular Devices) V max ) to monitor A 405 , and correlating it with time, thereby determining the timing and extent of fibrin lysis and clot formation. Clot formation leads to increased turbidity, manifested as A 405 Increase. This was confirmed by visual inspection at the end of each experiment. Clotting time is defined as reaching 1 / 2 the maximum ΔA 405 time. All data points are plotted as mean±SD of triplicate wells (n=3).
[0177] Und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com