Method of proliferating LAK cell
A cell and proliferation factor technology, applied in the field of LAK cell proliferation, can solve the problems of lymphocyte therapy not being effective, not showing antigen specificity, MHC binding, and expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
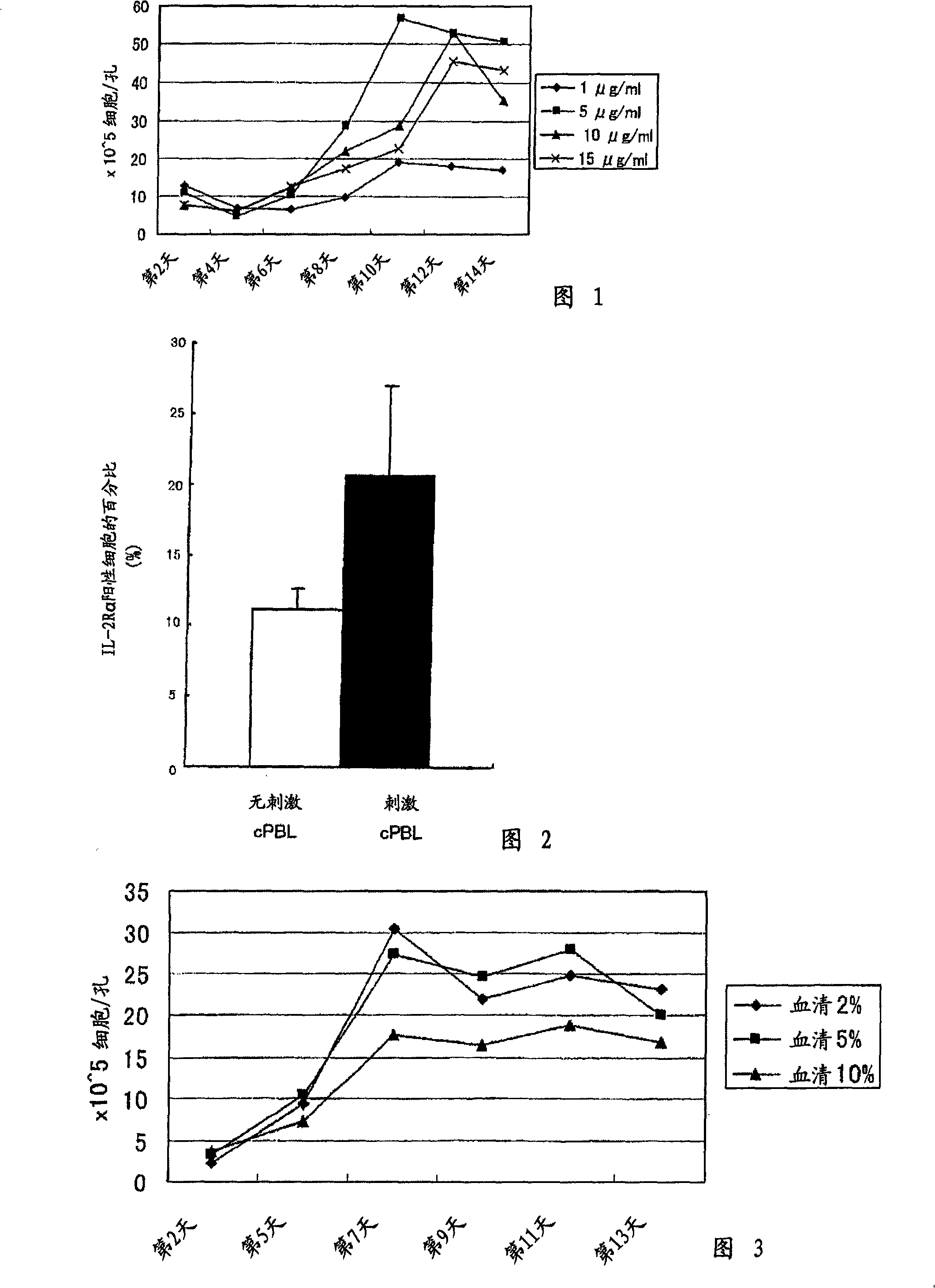
[0087] Using concanavalin A as a lectin and human interleukin-2 as a growth factor having interleukin-2-like activity, the optimal growth conditions of LAK cells were investigated.
[0088] 1. Optimization of Concanavalin A Concentration
[0089] (1) The collection of initial samples
[0090] 30 ml of whole blood (prevented with heparin from clotting) was drawn from the jugular vein of a doggy (6 years old) with a syringe. After centrifugation, add 10 times the amount of (a) NH 4 Cl buffer (NH 4 Cl: 0.83g / 100ml distilled water), (b) Tris base (20.6g / 1000ml distilled water, pH7.2), (c) (a) and (b) described in (c) mixed in a 9:1 filter-sterilized solution , placed in ice for 5 minutes (stirring frequently) to rupture the red blood cells contained in the blood.
[0091] 3 ml of specific gravity solution (for canine lymphocyte separation, lymphocyte (Lympholyte) manufactured by Cedarlane Co., Ltd.) was evenly put into 2 centrifuge tubes, and the ruptured blood was slowly layere...
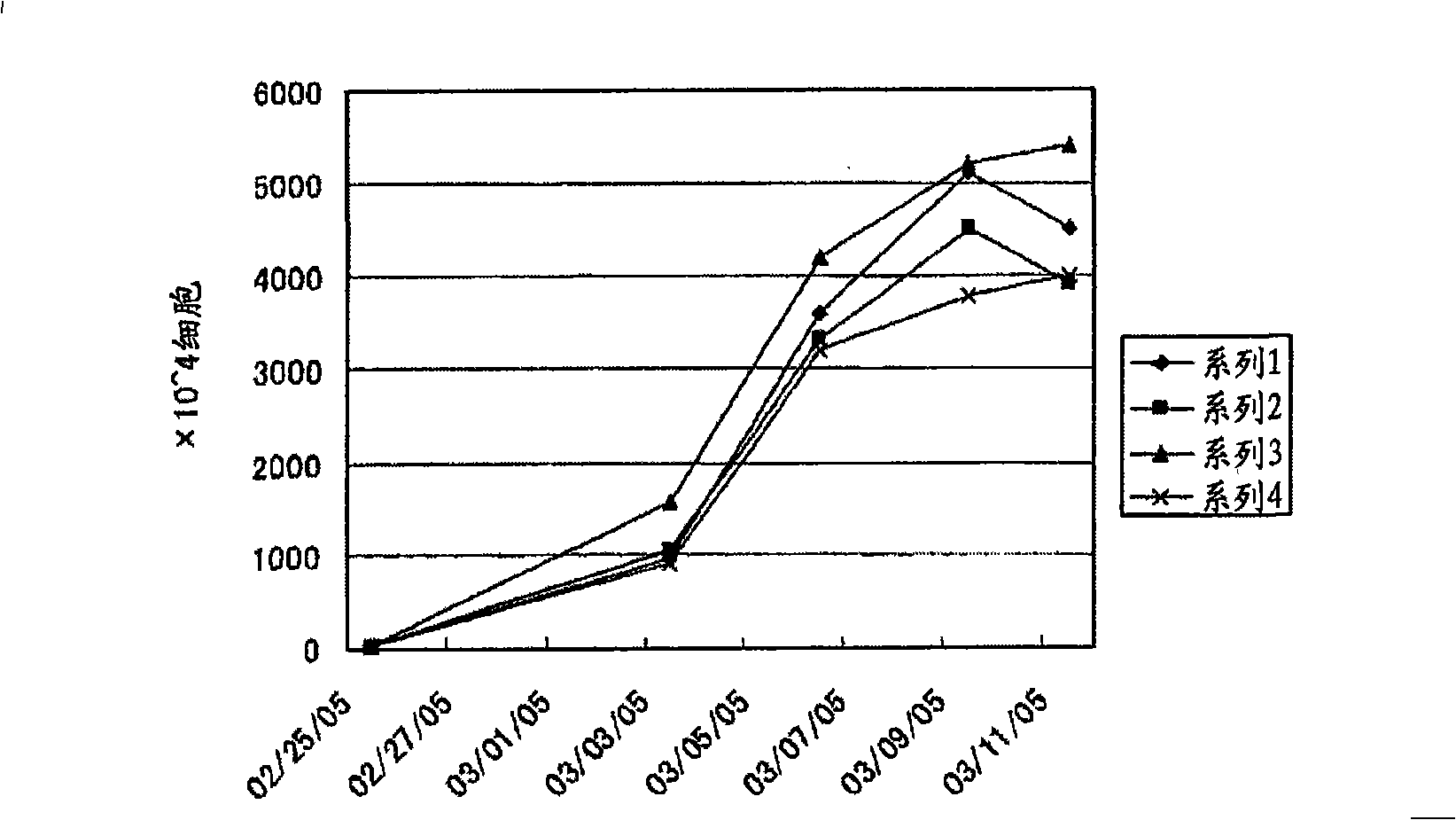
Embodiment 2
[0128] The effect of different sera used on the proliferation of LAK cells was investigated by changing the sera used to autoserum, fetal bovine serum, and cat serum. Specifically, follow the procedures below to investigate.
[0129] 1. When using your own serum
[0130] (1) Experimental method
[0131] By the same method as in Example 1, 1 ml of 4 systems were obtained from 4 dogs (Miniature Pinscher, 2 years old; Hybrid, 1 year old; Pomeranian, 10 years old; Hybrid, 3 years old) Cell suspension (cell density: 1.5×10 6 cells / ml). Add culture medium to the obtained cell suspensions of each system to adjust the number of cells to 1×10 5 cells / ml, add 3ml (3×10 5cells / well), Concanavalin A at a final concentration of 5 μg / ml, autoserum at a final concentration of 5%, and interleukin-2 at a final concentration of 750 U / ml were added respectively. In addition, in the same manner as in Example 1, the number of cells at the start day of cell proliferation was measured using a ...
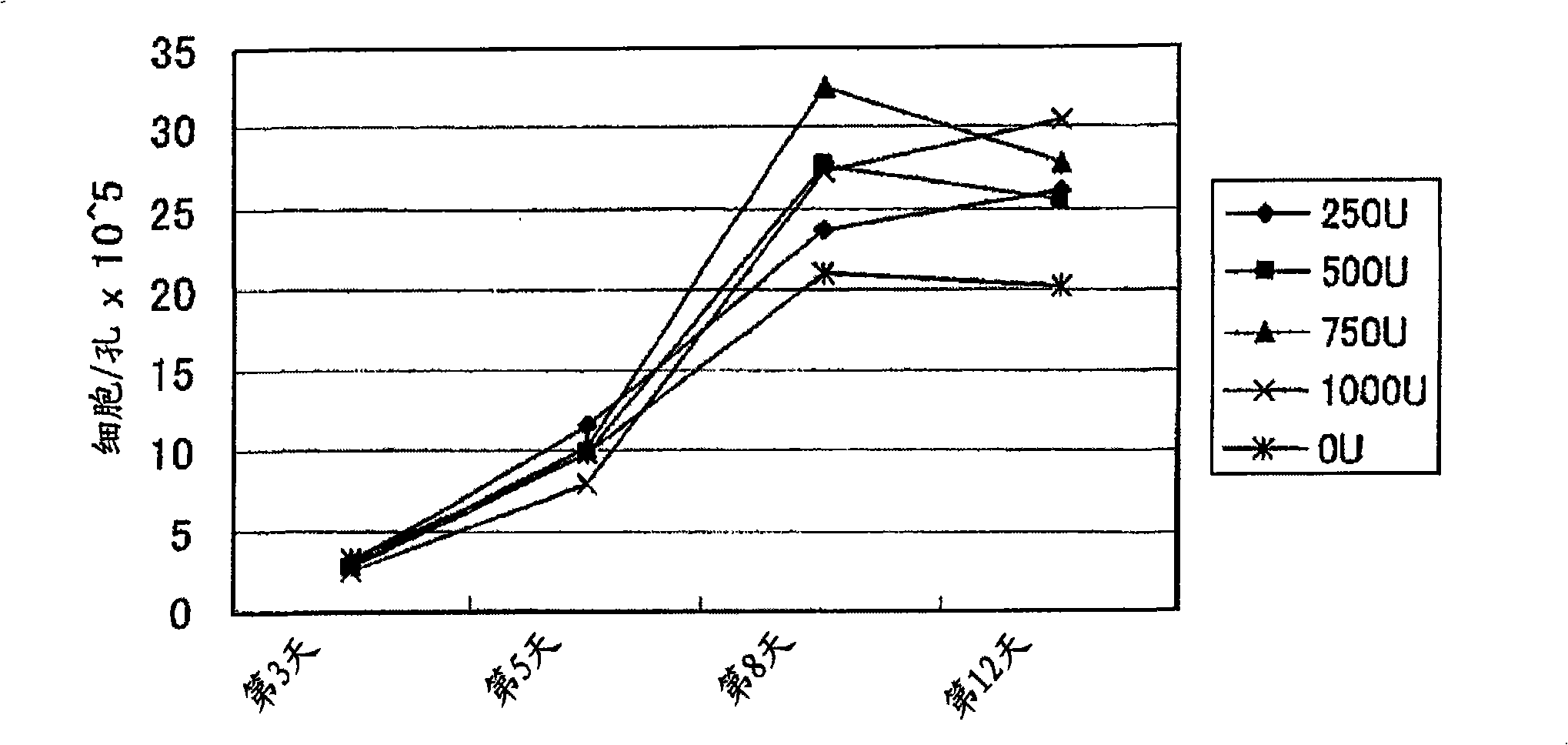
Embodiment 3
[0157] Changes in cell distribution due to proliferation were investigated by flow cytometry. Specifically, by the proliferation method of the present invention, for CD8 cells related to cellular immunity as αβ T cells + Cellular and CD4 associated with humoral immunity + The number of cells and the change in the ratio of the cells were investigated as follows.
[0158] (1) Experimental method
[0159] By the same method as in Example 1, 5 ml of cell suspension (cell density: 2~4×10) of 3 systems (for example, case) were obtained from 3 dogs (6 years old). 7 cells / ml). Add culture medium to the obtained cell suspensions of each system to adjust the number of cells to 1×10 6 Cells / ml, add 2ml per well (2×10 6 cells / well), add concanavalin A with a final concentration of 5 μg / ml, canine serum with a final concentration of 5% (taken from a dog for blood transfusion), fetal bovine serum with a final concentration of 10%, and concanavalin A with a final concentration of 750U / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com