Preparation method of canavaline polymer hydrogel, prepared hydrogel and application thereof
A technology of concanavalin and polymers, which is applied in the field of polymer materials and can solve problems such as undisclosed hydrogel adhesion properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
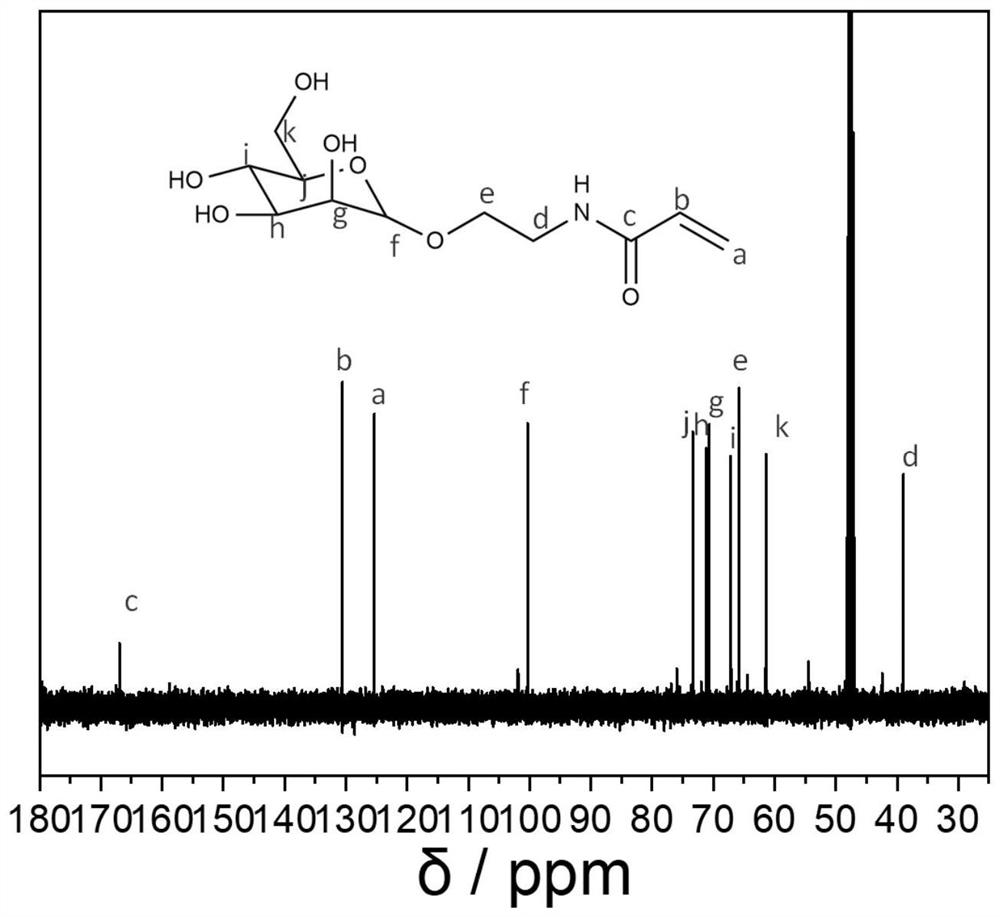
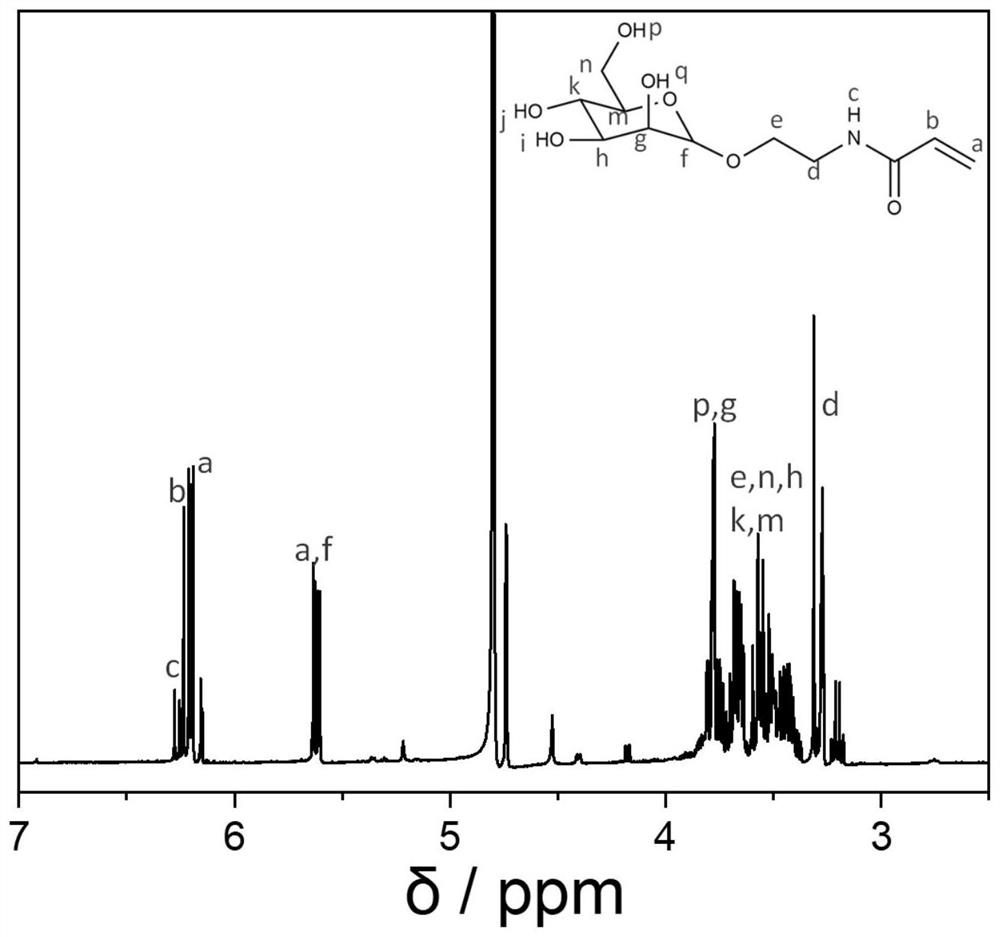
[0069] Preparation of Mannose Monomer
[0070] (1) Preparation of mannose-bromide: 3g of D-mannose (dried in vacuum at 60°C for 2 hours before use of mannose) and 150mg of sulfuric acid-silicon dioxide were added to a round-bottomed flask with a magnet, Then 9 mL of propynyl alcohol was added, heated at 60°C for 6 h, and then purified by a silica gel column using a developing solvent of methanol and dichloromethane with a volume of 6:1. Mannitol-bromine was obtained as 2.6 g of a yellow oily substance. The preparation of mannose-bromine is a typical substitution reaction, and the reaction conversion rate is very high with the participation of a catalyst. Some monomers that have not been successfully substituted can be separated and purified by using a silica gel chromatography column to obtain pure mannose-bromine monomer . Wherein the sulfuric acid-silicon dioxide catalyst is a prior art, which is a mixture of sulfuric acid and silicon dioxide in granular form, wherein the ...
Embodiment 2
[0085] Preparation of photodiacetylamide
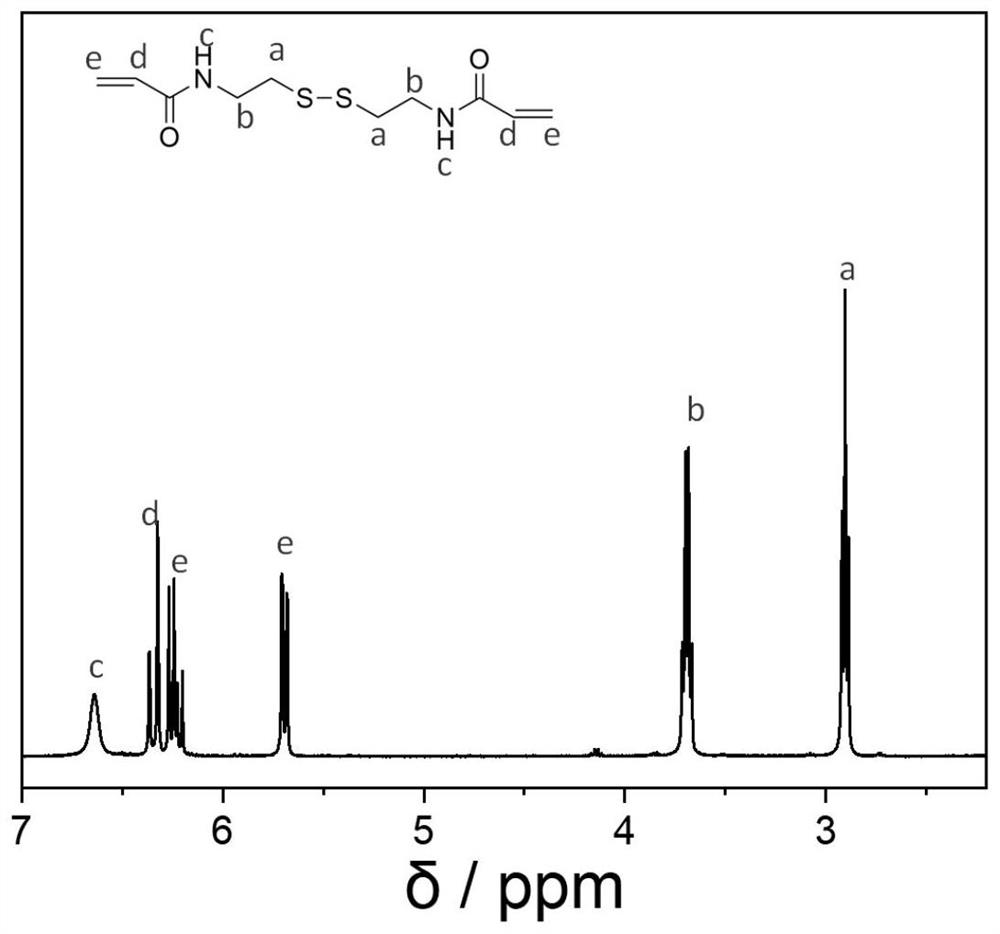
[0086] Dissolve 1 g of cystamine dihydrochloride in 20 mL of dry dichloromethane, then add 2.25 g of triethylamine, then slowly add 4 g of acryloyl chloride dropwise under stirring in an ice bath, after the dropwise addition is complete. React at room temperature for 2 h, then filter, and use ethyl acetate to separate through a silica gel column. The photodiacetamide was obtained as 0.5 g of white solid with a yield of 45%. image 3 It is the NMR spectrum of diacetamide.
[0087] The reaction equation is as follows:
[0088]
Embodiment 3
[0090] Preparation of HEPES buffer solution
[0091] The concentration of HEPES is 100mmol / L, the concentration of sodium chloride is 400mmol / L, the pH value of the solution is 7.2, the concentration of manganese chloride is 1mmol / L, and the concentration of calcium chloride is 1mmol / L.
[0092] When configuring HEPES buffer solution, mix HEPES and sodium chloride solution, adjust the pH value of the solution, and then add manganese chloride and calcium chloride, otherwise precipitation will occur.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com